Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody
- PMID: 33495307
- PMCID: PMC7963221
- DOI: 10.1126/science.abf4830
Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody
Abstract
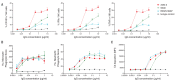
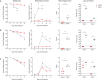
The recurrent zoonotic spillover of coronaviruses (CoVs) into the human population underscores the need for broadly active countermeasures. We employed a directed evolution approach to engineer three severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies for enhanced neutralization breadth and potency. One of the affinity-matured variants, ADG-2, displays strong binding activity to a large panel of sarbecovirus receptor binding domains and neutralizes representative epidemic sarbecoviruses with high potency. Structural and biochemical studies demonstrate that ADG-2 employs a distinct angle of approach to recognize a highly conserved epitope that overlaps the receptor binding site. In immunocompetent mouse models of SARS and COVID-19, prophylactic administration of ADG-2 provided complete protection against respiratory burden, viral replication in the lungs, and lung pathology. Altogether, ADG-2 represents a promising broad-spectrum therapeutic candidate against clade 1 sarbecoviruses.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Comment in
-
Exploiting a chink in the armor: engineering broadly neutralizing monoclonal antibodies for SARS-like viruses.Signal Transduct Target Ther. 2021 Jun 11;6(1):232. doi: 10.1038/s41392-021-00661-w. Signal Transduct Target Ther. 2021. PMID: 34117219 Free PMC article. No abstract available.
References
-
- Oude Munnink B. B., Sikkema R. S., Nieuwenhuijse D. F., Molenaar R. J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam-Blans M. C. A., Bouwstra R. J., GeurtsvanKessel C., van der Eijk A. A., Velkers F. C., Smit L. A. M., Stegeman A., van der Poel W. H. M., Koopmans M. P. G., Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371, 172–177 (2021). 10.1126/science.abe5901 - DOI - PMC - PubMed
-
- Wec A. Z., Wrapp D., Herbert A. S., Maurer D. P., Haslwanter D., Sakharkar M., Jangra R. K., Dieterle M. E., Lilov A., Huang D., Tse L. V., Johnson N. V., Hsieh C.-L., Wang N., Nett J. H., Champney E., Burnina I., Brown M., Lin S., Sinclair M., Johnson C., Pudi S., Bortz R. 3rd, Wirchnianski A. S., Laudermilch E., Florez C., Fels J. M., O’Brien C. M., Graham B. S., Nemazee D., Burton D. R., Baric R. S., Voss J. E., Chandran K., Dye J. M., McLellan J. S., Walker L. M., Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 369, 731–736 (2020). 10.1126/science.abc7424 - DOI - PMC - PubMed
-
- Pinto D., Park Y.-J., Beltramello M., Walls A. C., Tortorici M. A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J. B., Chen R. E., Havenar-Daughton C., Snell G., Telenti A., Virgin H. W., Lanzavecchia A., Diamond M. S., Fink K., Veesler D., Corti D., Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020). 10.1038/s41586-020-2349-y - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

