Arsenic trioxide replacing or reducing chemotherapy in consolidation therapy for acute promyelocytic leukemia (APL2012 trial)
- PMID: 33495363
- PMCID: PMC8017727
- DOI: 10.1073/pnas.2020382118
Arsenic trioxide replacing or reducing chemotherapy in consolidation therapy for acute promyelocytic leukemia (APL2012 trial)
Abstract
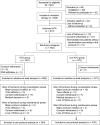
As all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are widely accepted in treating acute promyelocytic leukemia (APL), deescalating toxicity becomes a research hotspot. Here, we evaluated whether chemotherapy could be replaced or reduced by ATO in APL patients at different risks. After achieving complete remission with ATRA-ATO-based induction therapy, patients were randomized (1:1) into ATO and non-ATO groups for consolidation: ATRA-ATO versus ATRA-anthracycline for low-/intermediate-risk patients, or ATRA-ATO-anthracycline versus ATRA-anthracycline-cytarabine for high-risk patients. The primary end point was to assess disease-free survival (DFS) at 3 y by a noninferiority margin of -5%; 855 patients were enrolled with a median follow-up of 54.9 mo, and 658 of 755 patients could be evaluated at 3 y. In the ATO group, 96.1% (319/332) achieved 3-y DFS, compared to 92.6% (302/326) in the non-ATO group. The difference was 3.45% (95% CI -0.07 to 6.97), confirming noninferiority (P < 0.001). Using the Kaplan-Meier method, the estimated 7-y DFS was 95.7% (95% CI 93.6 to 97.9) in ATO and 92.6% (95% CI 89.8 to 95.4) in non-ATO groups (P = 0.066). Concerning secondary end points, the 7-y cumulative incidence of relapse (CIR) was significantly lower in ATO (2.2% [95% CI 1.1 to 4.2]) than in non-ATO group (6.1% [95% CI 3.9 to 9.5], P = 0.011). In addition, grade 3 to 4 hematological toxicities were significantly reduced in the ATO group during consolidation. Hence, ATRA-ATO in both chemotherapy-replacing and -reducing settings in consolidation is not inferior to ATRA-chemotherapy (https://www.clinicaltrials.gov/, NCT01987297).
Keywords: acute promyelocytic leukemia; all-trans retinoic acid; arsenic trioxide; consolidation therapy; risk stratification.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Adès L., et al. ., Treatment of newly diagnosed acute promyelocytic leukemia (APL): A comparison of French-Belgian-Swiss and PETHEMA results. Blood 111, 1078–1084 (2008). - PubMed
-
- Wang Z. Y., Chen Z., Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 111, 2505–2515 (2008). - PubMed
-
- Sanz M. A.et al. .; PETHEMA and HOVON Groups , Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood 115, 5137–5146 (2010). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

