SCA-1 micro-heterogeneity in the fate decision of dystrophic fibro/adipogenic progenitors
- PMID: 33495447
- PMCID: PMC7835386
- DOI: 10.1038/s41419-021-03408-1
SCA-1 micro-heterogeneity in the fate decision of dystrophic fibro/adipogenic progenitors
Abstract
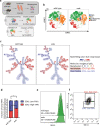
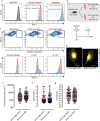
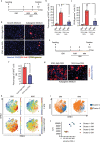
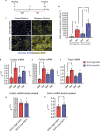
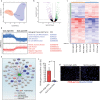
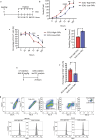
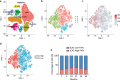
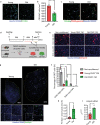
The term micro-heterogeneity refers to non-genetic cell to cell variability observed in a bell-shaped distribution of the expression of a trait within a population. The contribution of micro-heterogeneity to physiology and pathology remains largely uncharacterised. To address such an issue, we investigated the impact of heterogeneity in skeletal muscle fibro/adipogenic progenitors (FAPs) isolated from an animal model of Duchenne muscular dystrophy (DMD), the mdx mouse. FAPs play an essential role in muscle homoeostasis. However, in pathological conditions or ageing, they are the source of intramuscular infiltrations of fibrotic or adipose tissue. By applying a multiplex flow cytometry assay, we characterised and purified from mdx muscles two FAP cell states expressing different levels of SCA-1. The two cell states are morphologically identical and repopulate each other after several growth cycles. However, they differ in their in vitro behaviour. Cells expressing higher levels of SCA-1 (SCA1-High-FAPs) differentiate more readily into adipocytes while, when exposed to a fibrogenic stimulation, increase the expression of Col1a1 and Timp1 mRNA. A transcriptomic analysis confirmed the adipogenic propensity of SCA1-High-FAPs. In addition, SCA1-High-FAPs proliferate more extensively ex vivo and display more proliferating cells in dystrophic muscles in comparison to SCA1-Low-FAPs. Adipogenesis of both FAP cell states is inhibited in vitro by leucocytes from young dystrophic mice, while leucocytes isolated from aged dystrophic mice are less effective in limiting the adipogenesis of SCA1-High-FAPs suggesting a differential regulatory effect of the microenvironment on micro-heterogeneity. Our data suggest that FAP micro-heterogeneity is modulated in pathological conditions and that this heterogeneity in turn may impact on the behaviour of interstitial mesenchymal cells in genetic diseases.
Conflict of interest statement
The authors declare that they have no conflict of interest
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

