Novel myostatin-specific antibody enhances muscle strength in muscle disease models
- PMID: 33495503
- PMCID: PMC7835227
- DOI: 10.1038/s41598-021-81669-8
Novel myostatin-specific antibody enhances muscle strength in muscle disease models
Abstract
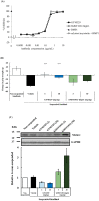
Myostatin, a member of the transforming growth factor-β superfamily, is an attractive target for muscle disease therapy because of its role as a negative regulator of muscle growth and strength. Here, we describe a novel antibody therapeutic approach that maximizes the potential of myostatin-targeted therapy. We generated an antibody, GYM329, that specifically binds the latent form of myostatin and inhibits its activation. Additionally, via "sweeping antibody technology", GYM329 reduces or "sweeps" myostatin in the muscle and plasma. Compared with conventional anti-myostatin agents, GYM329 and its surrogate antibody exhibit superior muscle strength-improvement effects in three different mouse disease models. We also demonstrate that the superior efficacy of GYM329 is due to its myostatin specificity and sweeping capability. Furthermore, we show that a GYM329 surrogate increases muscle mass in normal cynomolgus monkeys without any obvious toxicity. Our findings indicate the potential of GYM329 to improve muscle strength in patients with muscular disorders.
Conflict of interest statement
All authors are employees of Chugai Pharmaceutical Co., Ltd. Chugai Pharmaceutical Co., Ltd. is clinically developing GYM329.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

