Restoration of breathing after opioid overdose and spinal cord injury using temporal interference stimulation
- PMID: 33495588
- PMCID: PMC7835220
- DOI: 10.1038/s42003-020-01604-x
Restoration of breathing after opioid overdose and spinal cord injury using temporal interference stimulation
Abstract
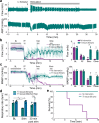
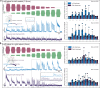
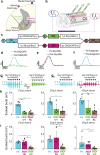
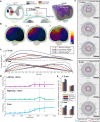
Respiratory insufficiency is a leading cause of death due to drug overdose or neuromuscular disease. We hypothesized that a stimulation paradigm using temporal interference (TI) could restore breathing in such conditions. Following opioid overdose in rats, two high frequency (5000 Hz and 5001 Hz), low amplitude waveforms delivered via intramuscular wires in the neck immediately activated the diaphragm and restored ventilation in phase with waveform offset (1 Hz or 60 breaths/min). Following cervical spinal cord injury (SCI), TI stimulation via dorsally placed epidural electrodes uni- or bilaterally activated the diaphragm depending on current and electrode position. In silico modeling indicated that an interferential signal in the ventral spinal cord predicted the evoked response (left versus right diaphragm) and current-ratio-based steering. We conclude that TI stimulation can activate spinal motor neurons after SCI and prevent fatal apnea during drug overdose by restoring ventilation with minimally invasive electrodes.
Conflict of interest statement
N.G. and E.S.B. have a patent on TI technology, assigned to MIT. E.N. is a board member and shareholder of TI Solutions AG, which develops stimulation devices and treatment-planning tools for temporal interference research. M.D.S., K.J.O., and D.D.F. have submitted a patent application related to use of TI for stimulating breathing. T.H.M. and A.M.C. declare no competing interests.
Figures


Similar articles
-
Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury.J Neurophysiol. 2017 Feb 1;117(2):767-776. doi: 10.1152/jn.00721.2016. Epub 2016 Nov 23. J Neurophysiol. 2017. PMID: 27881723 Free PMC article.
-
Rostral-Caudal Effect of Cervical Magnetic Stimulation on the Diaphragm Motor Evoked Potential after Cervical Spinal Cord Contusion in the Rat.J Neurotrauma. 2022 May;39(9-10):683-700. doi: 10.1089/neu.2021.0403. Epub 2022 Jan 26. J Neurotrauma. 2022. PMID: 34937419
-
Electrical stimulation of the sciatic nerve restores inspiratory diaphragm function in mice after spinal cord injury.Front Neural Circuits. 2025 Jan 22;18:1480291. doi: 10.3389/fncir.2024.1480291. eCollection 2024. Front Neural Circuits. 2025. PMID: 39911754 Free PMC article.
-
Restoration of respiratory muscle function following spinal cord injury. Review of electrical and magnetic stimulation techniques.Respir Physiol Neurobiol. 2005 Jul 28;147(2-3):273-87. doi: 10.1016/j.resp.2005.03.007. Respir Physiol Neurobiol. 2005. PMID: 16046197 Review.
-
Diaphragm Pacing.Clin Chest Med. 2018 Jun;39(2):459-471. doi: 10.1016/j.ccm.2018.01.008. Clin Chest Med. 2018. PMID: 29779603 Review.
Cited by
-
Non-invasive Modulation of Deep Brain Nuclei by Temporal Interference Stimulation.Neurosci Bull. 2025 May;41(5):853-865. doi: 10.1007/s12264-025-01359-7. Epub 2025 Feb 8. Neurosci Bull. 2025. PMID: 39920435
-
Cervical Epidural Electrical Stimulation Increases Respiratory Activity through Somatostatin-Expressing Neurons in the Dorsal Cervical Spinal Cord in Rats.J Neurosci. 2023 Jan 18;43(3):419-432. doi: 10.1523/JNEUROSCI.1958-21.2022. Epub 2022 Dec 7. J Neurosci. 2023. PMID: 36639888 Free PMC article.
-
[Research progress on transcranial electrical stimulation for deep brain stimulation].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2023 Oct 25;40(5):1005-1011. doi: 10.7507/1001-5515.202210012. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2023. PMID: 37879931 Free PMC article. Review. Chinese.
-
Electrical epidural stimulation of the cervical spinal cord: implications for spinal respiratory neuroplasticity after spinal cord injury.J Neurophysiol. 2021 Aug 1;126(2):607-626. doi: 10.1152/jn.00625.2020. Epub 2021 Jul 7. J Neurophysiol. 2021. PMID: 34232771 Free PMC article. Review.
-
A novel non-invasive brain stimulation technique: "Temporally interfering electrical stimulation".Front Neurosci. 2023 Jan 27;17:1092539. doi: 10.3389/fnins.2023.1092539. eCollection 2023. Front Neurosci. 2023. PMID: 36777641 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

