An Alveolata secretory machinery adapted to parasite host cell invasion
- PMID: 33495622
- PMCID: PMC8886610
- DOI: 10.1038/s41564-020-00854-z
An Alveolata secretory machinery adapted to parasite host cell invasion
Abstract
Apicomplexa are unicellular eukaryotes and obligate intracellular parasites, including Plasmodium (the causative agent of malaria) and Toxoplasma (one of the most widespread zoonotic pathogens). Rhoptries, one of their specialized secretory organelles, undergo regulated exocytosis during invasion1. Rhoptry proteins are injected directly into the host cell to support invasion and subversion of host immune function2. The mechanism by which they are discharged is unclear and appears distinct from those in bacteria, yeast, animals and plants. Here, we show that rhoptry secretion in Apicomplexa shares structural and genetic elements with the exocytic machinery of ciliates, their free-living relatives. Rhoptry exocytosis depends on intramembranous particles in the shape of a rosette embedded into the plasma membrane of the parasite apex. Formation of this rosette requires multiple non-discharge (Nd) proteins conserved and restricted to Ciliata, Dinoflagellata and Apicomplexa that together constitute the superphylum Alveolata. We identified Nd6 at the site of exocytosis in association with an apical vesicle. Sandwiched between the rosette and the tip of the rhoptry, this vesicle appears as a central element of the rhoptry secretion machine. Our results describe a conserved secretion system that was adapted to provide defence for free-living unicellular eukaryotes and host cell injection in intracellular parasites.
Conflict of interest statement
Competing interests
The authors declare no competing financial interests.
Figures


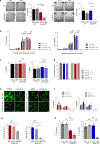
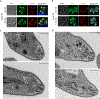




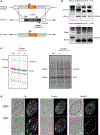






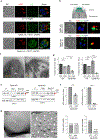


Comment in
-
The art of the steal.Nat Microbiol. 2021 Apr;6(4):421-422. doi: 10.1038/s41564-021-00886-z. Nat Microbiol. 2021. PMID: 33782557 No abstract available.
References
-
- Dubremetz JF Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell Microbiol 9, 841–8 (2007). - PubMed
-
- Boothroyd JC & Dubremetz JF Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol 6, 79–88 (2008). - PubMed
-
- Carruthers VB & Sibley LD Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73, 114–23 (1997). - PubMed
-
- Frenal K, Dubremetz JF, Lebrun M & Soldati-Favre D Gliding motility powers invasion and egress in Apicomplexa. Nat Rev Microbiol 15, 645–660 (2017). - PubMed
-
- Besteiro S, Dubremetz JF & Lebrun M The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol 13, 797–805 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

