Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption
- PMID: 33495635
- PMCID: PMC7933121
- DOI: 10.1038/s41593-020-00772-7
Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption
Abstract
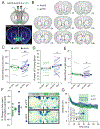
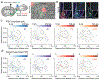
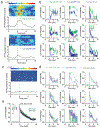
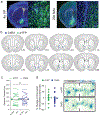
The nucleus accumbens shell (NAcSh) and the ventral pallidum (VP) are critical for reward processing, although the question of how coordinated activity within these nuclei orchestrates reward valuation and consumption remains unclear. Inhibition of NAcSh firing is necessary for reward consumption, but the source of this inhibition remains unknown. Here, we report that a subpopulation of VP neurons, the ventral arkypallidal (vArky) neurons, project back to the NAcSh, where they inhibit NAcSh neurons in vivo in mice. Consistent with this pathway driving reward consumption via inhibition of the NAcSh, calcium activity of vArky neurons scaled with reward palatability (which was dissociable from reward seeking) and predicted the subsequent drinking behavior during a free-access paradigm. Activation of the VP-NAcSh pathway increased ongoing reward consumption while amplifying hedonic reactions to reward. These results establish a pivotal role for vArky neurons in the promotion of reward consumption through modulation of NAcSh firing in a value-dependent manner.
Conflict of interest statement
Figures













Comment in
-
'Feedback' for feeding.Nat Neurosci. 2021 Mar;24(3):293-294. doi: 10.1038/s41593-021-00799-4. Nat Neurosci. 2021. PMID: 33547449 No abstract available.
References
Methods-only References:
-
- Paxinos G & Franklin BJF The Mouse Brain in Stereotaxic Coordinates Compact Fifth Edition (Academic, San Diego, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

