Ferroptosis: mechanisms, biology and role in disease
- PMID: 33495651
- PMCID: PMC8142022
- DOI: 10.1038/s41580-020-00324-8
Ferroptosis: mechanisms, biology and role in disease
Abstract
The research field of ferroptosis has seen exponential growth over the past few years, since the term was coined in 2012. This unique modality of cell death, driven by iron-dependent phospholipid peroxidation, is regulated by multiple cellular metabolic pathways, including redox homeostasis, iron handling, mitochondrial activity and metabolism of amino acids, lipids and sugars, in addition to various signalling pathways relevant to disease. Numerous organ injuries and degenerative pathologies are driven by ferroptosis. Intriguingly, therapy-resistant cancer cells, particularly those in the mesenchymal state and prone to metastasis, are exquisitely vulnerable to ferroptosis. As such, pharmacological modulation of ferroptosis, via both its induction and its inhibition, holds great potential for the treatment of drug-resistant cancers, ischaemic organ injuries and other degenerative diseases linked to extensive lipid peroxidation. In this Review, we provide a critical analysis of the current molecular mechanisms and regulatory networks of ferroptosis, the potential physiological functions of ferroptosis in tumour suppression and immune surveillance, and its pathological roles, together with a potential for therapeutic targeting. Importantly, as in all rapidly evolving research areas, challenges exist due to misconceptions and inappropriate experimental methods. This Review also aims to address these issues and to provide practical guidelines for enhancing reproducibility and reliability in studies of ferroptosis. Finally, we discuss important concepts and pressing questions that should be the focus of future ferroptosis research.
Figures
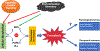
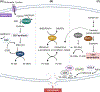
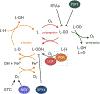

References
-
- Shen Q, Liang ML, Yang F, Deng YZ & Naqvi NI Ferroptosis contributes to developmental cell death in rice blast. New Phytol (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

