The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial
- PMID: 33495752
- PMCID: PMC7816625
- DOI: 10.1016/j.eclinm.2020.100720
The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial
Abstract
Background: Ivermectin inhibits the replication of SARS-CoV-2 in vitro at concentrations not readily achievable with currently approved doses. There is limited evidence to support its clinical use in COVID-19 patients. We conducted a Pilot, randomized, double-blind, placebo-controlled trial to evaluate the efficacy of a single dose of ivermectin reduce the transmission of SARS-CoV-2 when administered early after disease onset.
Methods: Consecutive patients with non-severe COVID-19 and no risk factors for complicated disease attending the emergency room of the Clínica Universidad de Navarra between July 31, 2020 and September 11, 2020 were enrolled. All enrollments occurred within 72 h of onset of fever or cough. Patients were randomized 1:1 to receive ivermectin, 400 mcg/kg, single dose (n = 12) or placebo (n = 12). The primary outcome measure was the proportion of patients with detectable SARS-CoV-2 RNA by PCR from nasopharyngeal swab at day 7 post-treatment. The primary outcome was supported by determination of the viral load and infectivity of each sample. The differences between ivermectin and placebo were calculated using Fisher's exact test and presented as a relative risk ratio. This study is registered at ClinicalTrials.gov: NCT04390022.
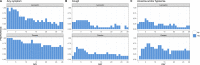
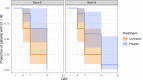
Findings: All patients recruited completed the trial (median age, 26 [IQR 19-36 in the ivermectin and 21-44 in the controls] years; 12 [50%] women; 100% had symptoms at recruitment, 70% reported headache, 62% reported fever, 50% reported general malaise and 25% reported cough). At day 7, there was no difference in the proportion of PCR positive patients (RR 0·92, 95% CI: 0·77-1·09, p = 1·0). The ivermectin group had non-statistically significant lower viral loads at day 4 (p = 0·24 for gene E; p = 0·18 for gene N) and day 7 (p = 0·16 for gene E; p = 0·18 for gene N) post treatment as well as lower IgG titers at day 21 post treatment (p = 0·24). Patients in the ivermectin group recovered earlier from hyposmia/anosmia (76 vs 158 patient-days; p < 0.001).
Interpretation: Among patients with non-severe COVID-19 and no risk factors for severe disease receiving a single 400 mcg/kg dose of ivermectin within 72 h of fever or cough onset there was no difference in the proportion of PCR positives. There was however a marked reduction of self-reported anosmia/hyposmia, a reduction of cough and a tendency to lower viral loads and lower IgG titers which warrants assessment in larger trials.
Funding: ISGlobal, Barcelona Institute for Global Health and Clínica Universidad de Navarra.
Keywords: Anosmia; COVID-19; Hyposmia; Ivermectin; SARS-CoV-2.
© 2021 The Author(s).
Conflict of interest statement
JLDP reports speaker fees from Pfizer and MSD as well as research grants from Novartis, outside the scope of the submitted work. No other competing interests were disclosed
Figures



References
-
- WHO. COVID-19 Weekly epidemiological update - 15 December 2020. Available at https://www.who.int/publications/m/item/weekly-epidemiological-update—15-december-2020 (Accessed December 21, 2020).
-
- Rubin R. Difficult to determine herd immunity threshold for COVID-19. JAMA. 2020;324(8):732. - PubMed
-
- WHO. Newsroom - coronavirus disease (COVID-19): vaccines. Available at https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-... (accessed December 21, 2020).
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

