Assessment of cochlear toxicity in response to chronic 3,3'-iminodipropionitrile in mice reveals early and reversible functional loss that precedes overt histopathology
- PMID: 33495873
- PMCID: PMC7904549
- DOI: 10.1007/s00204-020-02962-5
Assessment of cochlear toxicity in response to chronic 3,3'-iminodipropionitrile in mice reveals early and reversible functional loss that precedes overt histopathology
Abstract
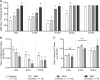
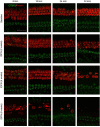
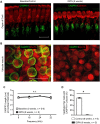
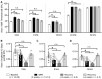
The peripheral auditory and vestibular systems rely on sensorineural structures that are vulnerable to ototoxic agents that cause hearing loss and/or equilibrium deficits. Although attention has focused on hair cell loss as the primary pathology underlying ototoxicity, evidence from the peripheral vestibular system indicates that hair cell loss during chronic exposure is preceded by synaptic uncoupling from the neurons and is potentially reversible. To determine if synaptic pathology also occurs in the peripheral auditory system, we examined the extent, time course, and reversibility of functional and morphological alterations in cochleae from mice exposed to 3,3'-iminodipropionitrile (IDPN) in drinking water for 2, 4 or 6 weeks. Functionally, IDPN exposure caused progressive high- to low-frequency hearing loss assessed by measurement of auditory brainstem response wave I absolute thresholds and amplitudes. The extent of hearing loss scaled with the magnitude of vestibular dysfunction assessed behaviorally. Morphologically, IDPN exposure caused progressive loss of outer hair cells (OHCs) and synapses between the inner hair cells (IHCs) and primary auditory neurons. In contrast, IHCs were spared from ototoxic damage. Importantly, hearing loss consistent with cochlear synaptopathy preceded loss of OHCs and synapses and, moreover, recovered if IDPN exposure was stopped before morphological pathology occurred. Our observations suggest that synaptic uncoupling, perhaps as an early phase of cochlear synaptopathy, also occurs in the peripheral auditory system in response to IDPN exposure. These findings identify novel mechanisms that contribute to the earliest stages of hearing loss in response to ototoxic agents and possibly other forms of acquired hearing loss.
Keywords: Acquired hearing loss; Afferent synapse; Cochlea; Cochlear synaptopathy; Hair cell; Ototoxicity; Vestibule.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

