Inherited Follicular Epithelial-Derived Thyroid Carcinomas: From Molecular Biology to Histological Correlates
- PMID: 33495912
- PMCID: PMC7960606
- DOI: 10.1007/s12022-020-09661-y
Inherited Follicular Epithelial-Derived Thyroid Carcinomas: From Molecular Biology to Histological Correlates
Abstract
Cancer derived from thyroid follicular epithelial cells is common; it represents the most common endocrine malignancy. The molecular features of sporadic tumors have been clarified in the past decade. However the incidence of familial disease has not been emphasized and is often overlooked in routine practice. A careful clinical documentation of family history or familial syndromes that can be associated with thyroid disease can help identify germline susceptibility-driven thyroid neoplasia. In this review, we summarize a large body of information about both syndromic and non-syndromic familial thyroid carcinomas. A significant number of patients with inherited non-medullary thyroid carcinomas manifest disease that appears to be sporadic disease even in some syndromic cases. The cytomorphology of the tumor(s), molecular immunohistochemistry, the findings in the non-tumorous thyroid parenchyma and other associated lesions may provide insight into the underlying syndromic disorder. However, the increasing evidence of familial predisposition to non-syndromic thyroid cancers is raising questions about the importance of genetics and epigenetics. What appears to be "sporadic" is becoming less often truly so and more often an opportunity to identify and understand novel genetic variants that underlie tumorigenesis. Pathologists must be aware of the unusual morphologic features that should prompt germline screening. Therefore, recognition of harbingers of specific germline susceptibility syndromes can assist in providing information to facilitate early detection to prevent aggressive disease.
Keywords: APC; Carney complex; Cowden syndrome; Cribriform-morular thyroid carcinoma; DICER1; DICER1 syndrome; FAP; Familial non-medullary thyroid carcinoma; PRKAR1A; PTEN; PTEN-hamartoma tumor syndrome; RASAL1; SDHB; Thyroid cancer; WRN; Wermer syndrome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
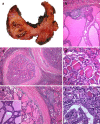

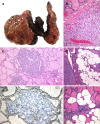
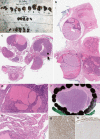
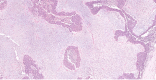
References
-
- Maxwell EL, Hall FT, Freeman JL. Familial non-medullary thyroid cancer: a matched-case control study. Laryngoscope. 2004;114(12):2182–2186. - PubMed
-
- Charkes ND. On the prevalence of familial nonmedullary thyroid cancer in multiply affected kindreds. Thyroid. 2006;16(2):181–186. - PubMed
-
- Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007;31(5):924–933. - PubMed
-
- Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, Inoue H, Kihara M, Higashiyama T, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145(1):100–105. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

