Effect of Mechanical Strain on Cells Involved in Fracture Healing
- PMID: 33496077
- PMCID: PMC7957396
- DOI: 10.1111/os.12885
Effect of Mechanical Strain on Cells Involved in Fracture Healing
Abstract
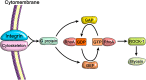
Secondary fracture healing is a complex multi-stage process in which the mechanical environment plays a key role. The use of an appropriate mechanical stimulation such as strain is conducive to tissue formation between fracture ends, thus aiding the healing process. However, if the strain is too large or too small, the biological behavior of the cells involved in bone healing will be affected, resulting in non-union or delayed healing. In this review, we summarize the current state of knowledge regarding the effect of strain on cells that play a role in the fracture-healing process. Overall, the related literature suggests that selection of an adequate strain promotes fracture healing through the stimulation of angiogenesis and osteogenesis, along with inhibition of osteoclast differentiation and bone resorption. However, standardized methods for the application of mechanical stimulation are lacking, and a unified consensus on the mechanism by which strain promotes cell differentiation has not yet been reached. These issues, therefore, deserve further investigation.
Keywords: Biomechanics; Fracture healing; Mesenchymal stem cell; Osteoblast; Osteoclast.
© 2021 The Authors. Orthopaedic Surgery published by Chinese Orthopaedic Association and John Wiley & Sons Australia, Ltd.
Figures


References
-
- Claes LE, Heigele CA, Neidlinger‐Wilke C, et al. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res, 1998, 355S: S132–S147. - PubMed
-
- Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res, 1997, 15: 577–584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

