Novel Guidewire Design and Coating for Continuous Delivery of Adenosine During Interventional Procedures
- PMID: 33496190
- PMCID: PMC7955438
- DOI: 10.1161/JAHA.120.019275
Novel Guidewire Design and Coating for Continuous Delivery of Adenosine During Interventional Procedures
Erratum in
-
Correction to: Novel Guidewire Design and Coating for Continuous Delivery of Adenosine During Interventional Procedures.J Am Heart Assoc. 2021 Feb 16;10(4):e014656. doi: 10.1161/JAHA.119.014656. Epub 2021 Feb 9. J Am Heart Assoc. 2021. PMID: 33559476 Free PMC article. No abstract available.
Abstract
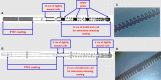
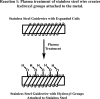
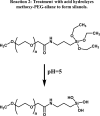
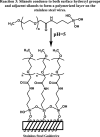
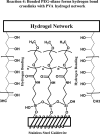
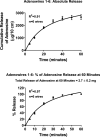
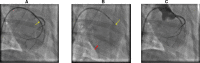
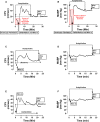
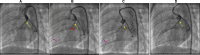
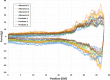
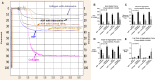
Background The "no-reflow phenomenon" compromises percutaneous coronary intervention outcomes. There is an unmet need for a device that prevents no-reflow phenomenon. Our goal was to develop a guidewire platform comprising a nondisruptive hydrophilic coating that allows continuous delivery of adenosine throughout a percutaneous coronary intervention. Methods and Results We developed a guidewire with spaced coils to increase surface area for drug loading. Guidewires were plasma treated to attach hydroxyl groups to metal surfaces, and a methoxy-polyethylene glycol-silanol primer layer was covalently linked to hydroxyl groups. Using polyvinyl alcohol, polyvinyl pyrrolidone, and polyvinyl acetate, a drug layer containing jet-milled adenosine was hydrogen-bonded to the polyethylene glycol-silanol layer and coated with an outer diffusive barrier layer. Coatings were processed with a freeze/thaw curing method. In vitro release studies were conducted followed by in vivo evaluation in pigs. Coating quality, performance, and stability with sterilization were also evaluated. Antiplatelet properties of the guidewire were also determined. Elution studies with adenosine-containing guidewires showed curvilinear and complete release of adenosine over 60 minutes. Porcine studies demonstrated that upon insertion into a coronary artery, adenosine-releasing guidewires induced immediate and robust increases (2.6-fold) in coronary blood flow velocity, which were sustained for ≈30 minutes without systemic hemodynamic effects or arrhythmias. Adenosine-loaded wires prevented and reversed coronary vasoconstriction induced by acetylcholine. The wires significantly inhibited platelet aggregation by >80% in vitro. Guidewires passed bench testing for lubricity, adherence, integrity, and tracking. Conclusions Our novel drug-releasing guidewire platform represents a unique approach to prevent/treat no-reflow phenomenon during percutaneous coronary intervention.
Keywords: adenosine; cardiac guidewire; no‐reflow phenomenon; percutaneous coronary intervention.
Conflict of interest statement
Drs Forman, Brewer, Brown, Menshikova, Lowman, and Jackson have equity ownership in Adenopaint, LLC, a company that is developing Adenowire for interventional cardiology.
Figures




References
-
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute infarction: a quantitative review of 23 randomized trials. Lancet. 2013;361:13–20. - PubMed
-
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction–related myocardial injury and coronary no‐reflow following percutaneous intervention: a systematic approach. JACC Cardiovasc Interv. 2010;3:695–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

