A streamlined whole blood CyTOF workflow defines a circulating immune cell signature of COVID-19
- PMID: 33496367
- PMCID: PMC8013522
- DOI: 10.1002/cyto.a.24317
A streamlined whole blood CyTOF workflow defines a circulating immune cell signature of COVID-19
Abstract
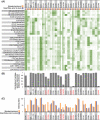
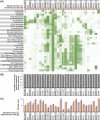
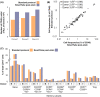
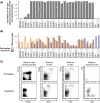
Mass cytometry (CyTOF) represents one of the most powerful tools in immune phenotyping, allowing high throughput quantification of over 40 parameters at single-cell resolution. However, wide deployment of CyTOF-based immune phenotyping studies are limited by complex experimental workflows and the need for specialized CyTOF equipment and technical expertise. Furthermore, differences in cell isolation and enrichment protocols, antibody reagent preparation, sample staining, and data acquisition protocols can all introduce technical variation that can confound integrative analyses of large data-sets of samples processed across multiple labs. Here, we present a streamlined whole blood CyTOF workflow which addresses many of these sources of experimental variation and facilitates wider adoption of CyTOF immune monitoring across sites with limited technical expertise or sample-processing resources or equipment. Our workflow utilizes commercially available reagents including the Fluidigm MaxPar Direct Immune Profiling Assay (MDIPA), a dry tube 30-marker immunophenotyping panel, and SmartTube Proteomic Stabilizer, which allows for simple and reliable fixation and cryopreservation of whole blood samples. We validate a workflow that allows for streamlined staining of whole blood samples with minimal processing requirements or expertise at the site of sample collection, followed by shipment to a central CyTOF core facility for batched downstream processing and data acquisition. We apply this workflow to characterize 184 whole blood samples collected longitudinally from a cohort of 72 hospitalized COVID-19 patients and healthy controls, highlighting dynamic disease-associated changes in circulating immune cell frequency and phenotype.
Keywords: COVID-19; CyTOF; human whole blood immunophenotyping; mass cytometry.
© 2021 International Society for Advancement of Cytometry.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures


Update of
-
A Streamlined CyTOF Workflow To Facilitate Standardized Multi-Site Immune Profiling of COVID-19 Patients.medRxiv [Preprint]. 2020 Jun 29:2020.06.26.20141341. doi: 10.1101/2020.06.26.20141341. medRxiv. 2020. Update in: Cytometry A. 2021 May;99(5):446-461. doi: 10.1002/cyto.a.24317. PMID: 32607524 Free PMC article. Updated. Preprint.
References
-
- Chan RC‐F, Kotner JS, Chuang CM‐H, Gaur A. Stabilization of pre‐optimized multicolor antibody cocktails for flow cytometry applications. Cytometry B Clin Cytom. 2017;92:508–24. - PubMed
-
- Bagwell CB, Hunsberger B, Hill B, Herbert D, Bray C, Selvanantham T, et al. Multi‐site reproducibility of a human immunophenotyping assay in whole blood and peripheral blood mononuclear cells preparations using CyTOF technology coupled with Maxpar Pathsetter, an automated data analysis system. Cytometry B Clin Cytom. 2020;98:146–60. - PMC - PubMed
-
- Charney AW, Simons NW, Mouskas K, Lepow L, Cheng E, Le Berichel J, et al. Sampling the host response to SARS‐CoV‐2 in hospitals under siege. Nat Med. 2020;26:1157–8. - PubMed
-
- Amir E‐AD, Guo XV, Mayovska O, Rahman AH. Average overlap frequency: a simple metric to evaluate staining quality and community identification in high dimensional mass cytometry experiments. J Immunol Methods. 2018;453:20–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

