Rapid removal of phagosomal ferroportin in macrophages contributes to nutritional immunity
- PMID: 33496744
- PMCID: PMC7839378
- DOI: 10.1182/bloodadvances.2020002833
Rapid removal of phagosomal ferroportin in macrophages contributes to nutritional immunity
Abstract
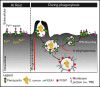
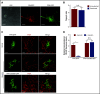
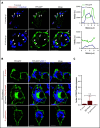
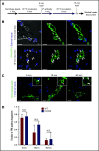
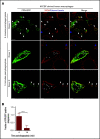
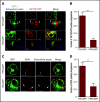
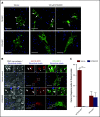
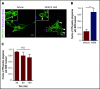
Nutrient sequestration is an essential facet of host innate immunity. Macrophages play a critical role in controlling iron availability through expression of the iron transport protein ferroportin (FPN), which extrudes iron from the cytoplasm to the extracellular milieu. During phagocytosis, the limiting phagosomal membrane, which derives from the plasmalemma, can be decorated with FPN and, if functional, will move iron from the cytosol into the phagosome lumen. This serves to feed iron to phagocytosed microbes and would be counterproductive to the many other known host mechanisms working to starve microbes of this essential metal. To understand how FPN is regulated during phagocytosis, we expressed FPN as a green fluorescent protein-fusion protein in macrophages and monitored its localization during uptake of various phagocytic targets, including Staphylococcus aureus, Salmonella enterica serovar Typhimurium, human erythrocytes, and immunoglobulin G opsonized latex beads. We find that FPN is rapidly removed, independently of Vps34 and PI(3)P, from early phagosomes and does not follow recycling pathways that regulate transferrin receptor recycling. Live-cell video microscopy showed that FPN movement on the phagosome is dynamic, with punctate and tubular structures forming before FPN is trafficked back to the plasmalemma. N-ethylmaleimide-sensitive factor, which disrupts soluble NSF attachment protein receptor (SNARE)-mediated membrane fusion and trafficking, prevented FPN removal from the phagosome. Our data support the hypothesis that removal of FPN from the limiting phagosomal membrane will, at the cellular level, ensure that iron cannot be pumped into phagosomes. We propose this as yet another mechanism of host nutritional immunity to subvert microbial growth.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Weinberg ED. Iron and susceptibility to infectious disease. Science. 1974;184(4140):952-956. - PubMed
-
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237-1248. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

