Adoptive therapy with CMV-specific cytotoxic T lymphocytes depends on baseline CD4+ immunity to mediate durable responses
- PMID: 33496746
- PMCID: PMC7839363
- DOI: 10.1182/bloodadvances.2020002735
Adoptive therapy with CMV-specific cytotoxic T lymphocytes depends on baseline CD4+ immunity to mediate durable responses
Abstract
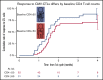
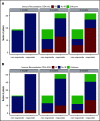
Adoptive cell therapy using cytomegalovirus (CMV)-specific cytotoxic T lymphocytes (CMV-CTLs) has demonstrated efficacy posttransplant. Despite the predicted limited engraftment of CMV-CTLs derived from third-party donors, partially matched third-party donor-derived CMV-CTLs have demonstrated similar response rates to those derived from primary hematopoietic cell transplantation donors. Little is known about the mechanisms through which adoptive cellular therapies mediate durable responses. We performed a retrospective analysis of patients receiving CMV-CTLs for treatment of CMV viremia and/or disease after allogeneic transplant between September of 2009 and January of 2018. We evaluated whether response to adoptively transferred CMV-CTLs correlated with immune reconstitution (IR), using validated CD4+ IR milestones of 50 × 106/L and 200 × 106/L. In this analysis, a cohort of 104 patients received CMV-CTLs derived from a primary transplant donor (n = 25), a third-party donor (n = 76), or both (n = 3). Response to therapy did not increase the likelihood of achieving CD4+ IR milestones at 1 (P = .53 and P > .99) or 2 months (P = .12 and P = .33). The origin of CMV-CTLs did not impact subsequent CD4+ IR. CMV-CTLs appeared to interact with host immunity in mediating responses. Recipients with a baseline CD4 >50 × 106/L had higher response to therapy (P = .02), improved overall survival (P < .001), and protection from CMV-related death (P = .002). Baseline endogenous immunity appears to improve CMV-related and overall survival in this cohort and can be an important marker at the initiation of therapy.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.J.O. and E.D. have received consulting fees and grant support from Atara Biotherapeutics, the Starr Cancer Consortium, and National Cancer Institute P01 CA23766. When Atara Biotherapeutics licensed banks of Epstein-Barr virus and CMV T cells, R.J.O. and E.D. received part of the royalties paid to MSKCC. MSKCC holds several patents related to this work on which R.J.O. and E.D. are inventors. S.E.P. receives support for the conduct of sponsored trials from Atara Biotherapeutics, Mesoblast, and Jasper. S.E.P. is an inventor of intellectual property licensed to Atara Biotherapeutics by MSKCC; S.E.P. assigned all rights to MSKCC and has no financial interest in Atara Biotherapeutics. The remaining authors declare no competing financial interests.
Figures


References
-
- Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143-149. - PubMed
-
- Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18(1):10-18. - PubMed
-
- Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467-480. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

