Integrative analysis of a phase 2 trial combining lenalidomide with CHOP in angioimmunoblastic T-cell lymphoma
- PMID: 33496747
- PMCID: PMC7839364
- DOI: 10.1182/bloodadvances.2020003081
Integrative analysis of a phase 2 trial combining lenalidomide with CHOP in angioimmunoblastic T-cell lymphoma
Abstract
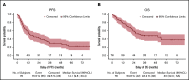
Angioimmunoblastic T-cell lymphoma (AITL) is a frequent T-cell lymphoma in the elderly population that has a poor prognosis when treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy. Lenalidomide, which has been safely combined with CHOP to treat B-cell lymphoma, has shown efficacy as a single agent in AITL treatment. We performed a multicentric phase 2 trial combining 25 mg lenalidomide daily for 14 days per cycle with 8 cycles of CHOP21 in previously untreated AITL patients aged 60 to 80 years. The primary objective was the complete metabolic response (CMR) rate at the end of treatment. Seventy-eight of the 80 patients enrolled were included in the efficacy and safety analysis. CMR was achieved in 32 (41%; 95% confidence interval [CI], 30%-52.7%) patients, which was below the prespecified CMR rate of 55% defined as success in the study. The 2-year progression-free survival (PFS) was 42.1% (95% CI, 30.9%-52.8%), and the 2-year overall survival was 59.2% (95% CI, 47.3%-69.3%). The most common toxicities were hematologic and led to treatment discontinuation in 15% of patients. This large prospective and uniform series of AITL treatment data was used to perform an integrative analysis of clinical, pathologic, biologic, and molecular data. TET2, RHOA, DNMT3A, and IDH2 mutations were present in 78%, 54%, 32%, and 22% of patients, respectively. IDH2 mutations were associated with distinct pathologic and clinical features and DNMT3A was associated with shorter PFS. In conclusion, the combination of lenalidomide and CHOP did not improve the CMR in AITL patients. This trial clarified the clinical impact of recurrent mutations in AITL. This trial was registered at www.clincialtrials.gov as #NCT01553786.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.C. has consulted for Roche and Celgene and received honoraria from Sanofi, Gilead, Jansen, AbbVie, Roche, and Celgene. C.H. reports personal fees from Celgene during the conduct of the study and personal fees from Roche, Amgen, Janssen, Gilead, Novartis, and Takeda outside the submitted work. The remaining authors declare no competing financial interests.
Figures



References
-
- Laurent C, Baron M, Amara N, et al. Impact of expert pathologic review of lymphoma diagnosis: study of patients from the French Lymphopath Network. J Clin Oncol. 2017;35(18):2008-2017. - PubMed
-
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project . International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. - PubMed
-
- de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010;148(5):673-689. - PubMed
-
- de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952-4963. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

