Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone-Sensitive Prostate Cancer: A Randomized Clinical Trial
- PMID: 33496795
- PMCID: PMC7838941
- DOI: 10.1001/jamanetworkopen.2020.34633
Clinical Efficacy of Enzalutamide vs Bicalutamide Combined With Androgen Deprivation Therapy in Men With Metastatic Hormone-Sensitive Prostate Cancer: A Randomized Clinical Trial
Abstract
Importance: Black patients have been underrepresented in prospective clinical trials of advanced prostate cancer. This study evaluated the efficacy of enzalutamide compared with bicalutamide, with planned subset analysis of Black patients with metastatic hormone-sensitive prostate cancer (mHSPC), which is a disease state responsive to androgen deprivation therapy (ADT).
Objective: To compare the efficacy of enzalutamide vs bicalutamide in combination with ADT in men with mHSPC, with a subset analysis of Black patients.
Design, setting, and participants: In this randomized clinical trial, a phase 2 screening design enabled a nondefinitive comparison of the primary outcome by treatment. Patients were stratified by race (Black or other) and bone pain (present or absent). Accrual of at least 30% Black patients was required. This multicenter trial was conducted at 4 centers in the US. Men with mHSPC with no history of seizures and adequate marrow, renal, and liver function were eligible. Data analysis was performed from February 2019 to March 2020.
Interventions: Participants were randomized 1:1 to receive oral enzalutamide (160 mg daily) or bicalutamide (50 mg daily) in addition to ADT.
Main outcomes and measures: The primary end point was the 7-month prostate-specific antigen (PSA) response (SMPR) rate, a previously accepted surrogate for overall survival (OS) outcome. Secondary end points included adverse reactions, time to PSA progression, and OS.
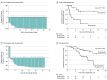
Results: A total of 71 men (median [range] age, 65 [51-86] years) were enrolled; 29 (41%) were Black, 41 (58%) were White, and 1 (1%) was Asian. Thirty-six patients were randomized to receive enzalutamide, and 35 were randomized to receive bicalutamide. Twenty-six patients (37%) had bone pain and 37 patients (52%) had extensive disease. SMPR was achieved in 30 of 32 patients (94%; 95% CI, 80%-98%) taking enzalutamide and 17 of 26 patients (65%; 95% CI, 46%-81%) taking bicalutamide (P = .008) (difference, 29%; 95% CI, 5%-50%). Among Black patients, the SMPR was 93% (95% CI, 69%-99%) among those taking enzalutamide and 42% (95% CI, 19%-68%) among those taking bicalutamide (P = .009); among non-Black patients, the SMPR was 94% (95% CI, 74%-99%) among those taking enzalutamide and 86% (95% CI, 60%-96%) among those taking bicalutamide. The 12-month PSA response rates were 84% with enzalutamide and 34% with bicalutamide.
Conclusions and relevance: The findings of this randomized clinical trial comparing enzalutamide with bicalutamide suggest that enzalutamide is associated with improved outcomes compared with bicalutamide, in terms of the rate and duration of PSA response, in Black patients with mHSPC.
Trial registration: ClinicalTrials.gov Identifier: NCT02058706.
Conflict of interest statement
Figures


Comment in
-
Successful Recruitment of Black Men to Prostate Cancer Clinical Trials-A Lesson in Achievement.JAMA Netw Open. 2021 Jan 4;4(1):e2034652. doi: 10.1001/jamanetworkopen.2020.34652. JAMA Netw Open. 2021. PMID: 33496790 No abstract available.
Similar articles
-
Enzalutamide and Prostate-Specific Antigen Levels in Metastatic Prostate Cancer: A Secondary Analysis of the ARCHES Randomized Clinical Trial.JAMA Netw Open. 2025 May 1;8(5):e258751. doi: 10.1001/jamanetworkopen.2025.8751. JAMA Netw Open. 2025. PMID: 40332939 Free PMC article. Clinical Trial.
-
Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study.Lancet Oncol. 2016 Feb;17(2):153-163. doi: 10.1016/S1470-2045(15)00518-5. Epub 2016 Jan 14. Lancet Oncol. 2016. PMID: 26774508 Clinical Trial.
-
Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial.J Clin Oncol. 2016 Jun 20;34(18):2098-106. doi: 10.1200/JCO.2015.64.9285. Epub 2016 Jan 25. J Clin Oncol. 2016. PMID: 26811535 Clinical Trial.
-
Evaluation of Fall and Fracture Risk Among Men With Prostate Cancer Treated With Androgen Receptor Inhibitors: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Nov 2;3(11):e2025826. doi: 10.1001/jamanetworkopen.2020.25826. JAMA Netw Open. 2020. PMID: 33201234 Free PMC article.
-
Enzalutamide for the treatment of nonmetastatic castration-resistant prostate cancer.Expert Opin Pharmacother. 2020 Dec;21(17):2091-2099. doi: 10.1080/14656566.2020.1803281. Epub 2020 Aug 12. Expert Opin Pharmacother. 2020. PMID: 32783772 Review.
Cited by
-
Androgen receptor axis-targeted agents are not superior to conventional hormonal therapy for treatment of metastatic prostate cancer.Oncol Lett. 2022 Aug 9;24(4):333. doi: 10.3892/ol.2022.13453. eCollection 2022 Oct. Oncol Lett. 2022. PMID: 36039059 Free PMC article.
-
Targeting Prostate Cancer, the 'Tousled Way'.Int J Mol Sci. 2023 Jul 5;24(13):11100. doi: 10.3390/ijms241311100. Int J Mol Sci. 2023. PMID: 37446279 Free PMC article. Review.
-
TRIM11 Posttranscriptionally Modulated by miR-5193 Facilitates Tumor Growth and Metastasis of Prostate Cancer.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231178639. doi: 10.1177/15330338231178639. Technol Cancer Res Treat. 2023. PMID: 37248611 Free PMC article.
-
Characterization of PSA dynamics and oncological outcomes in patients with metastatic hormone-sensitive prostate cancer treated with androgen receptor signaling inhibitors.Int J Clin Oncol. 2025 Mar;30(3):539-550. doi: 10.1007/s10147-024-02676-z. Epub 2024 Dec 10. Int J Clin Oncol. 2025. PMID: 39656401 Free PMC article.
-
Comparative effectiveness of multiple androgen receptor signaling inhibitor medicines with androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a study in the real world.Front Oncol. 2024 Apr 18;14:1324181. doi: 10.3389/fonc.2024.1324181. eCollection 2024. Front Oncol. 2024. PMID: 38699643 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

