Dysregulation of the NRG1/ERBB pathway causes a developmental disorder with gastrointestinal dysmotility in humans
- PMID: 33497358
- PMCID: PMC7954599
- DOI: 10.1172/JCI145837
Dysregulation of the NRG1/ERBB pathway causes a developmental disorder with gastrointestinal dysmotility in humans
Abstract
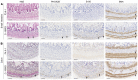
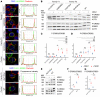
Hirschsprung disease (HSCR) is the most frequent developmental anomaly of the enteric nervous system, with an incidence of 1 in 5000 live births. Chronic intestinal pseudo-obstruction (CIPO) is less frequent and classified as neurogenic or myogenic. Isolated HSCR has an oligogenic inheritance with RET as the major disease-causing gene, while CIPO is genetically heterogeneous, caused by mutations in smooth muscle-specific genes. Here, we describe a series of patients with developmental disorders including gastrointestinal dysmotility, and investigate the underlying molecular bases. Trio-exome sequencing led to the identification of biallelic variants in ERBB3 and ERBB2 in 8 individuals variably associating HSCR, CIPO, peripheral neuropathy, and arthrogryposis. Thorough gut histology revealed aganglionosis, hypoganglionosis, and intestinal smooth muscle abnormalities. The cell type-specific ErbB3 and ErbB2 function was further analyzed in mouse single-cell RNA sequencing data and in a conditional ErbB3-deficient mouse model, revealing a primary role for ERBB3 in enteric progenitors. The consequences of the identified variants were evaluated using quantitative real-time PCR (RT-qPCR) on patient-derived fibroblasts or immunoblot assays on Neuro-2a cells overexpressing WT or mutant proteins, revealing either decreased expression or altered phosphorylation of the mutant receptors. Our results demonstrate that dysregulation of ERBB3 or ERBB2 leads to a broad spectrum of developmental anomalies, including intestinal dysmotility.
Keywords: Development; Gastroenterology; Genetic diseases; Molecular genetics.
Conflict of interest statement
Figures






Comment in
-
Hirschsprung disease and more: dysregulation of ERBB2 and ERBB3.J Clin Invest. 2021 Mar 15;131(6):e146389. doi: 10.1172/JCI146389. J Clin Invest. 2021. PMID: 33720042 Free PMC article.
References
-
- Hirschsprung H. Stuhlträgheit neugeborener in folge von dilatation und hypertrophie des colons. Jahrbuch Kinderheilkunde Physische Erziehung. 1888;27:1–7.
-
- Amiel J, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45(1):1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

