Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine
- PMID: 33497364
- PMCID: PMC7934923
- DOI: 10.1172/jci.insight.141088
Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine
Abstract
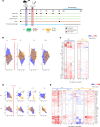
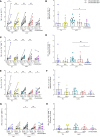
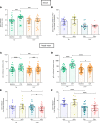
Influenza virus infections affect millions of people annually, and current available vaccines provide varying rates of protection. However, the way in which the nasal microbiota, particularly established pneumococcal colonization, shape the response to influenza vaccination is not yet fully understood. In this study, we inoculated healthy adults with live Streptococcus pneumoniae and vaccinated them 3 days later with either tetravalent-inactivated influenza vaccine (TIV) or live attenuated influenza vaccine (LAIV). Vaccine-induced immune responses were assessed in nose, blood, and lung. Nasal pneumococcal colonization had no impact upon TIV-induced antibody responses to influenza, which manifested in all compartments. However, experimentally induced pneumococcal colonization dampened LAIV-mediated mucosal antibody responses, primarily IgA in the nose and IgG in the lung. Pulmonary influenza-specific cellular responses were more apparent in the LAIV group compared with either the TIV or an unvaccinated group. These results indicate that TIV and LAIV elicit differential immunity to adults and that LAIV immunogenicity is diminished by the nasal presence of S. pneumoniae. Therefore, nasopharyngeal pneumococcal colonization may affect LAIV efficacy.
Keywords: Adaptive immunity; Immunology; Influenza; Vaccines.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

