Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits?
- PMID: 33497588
- PMCID: PMC7881176
- DOI: 10.1098/rsob.200352
Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits?
Abstract
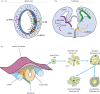
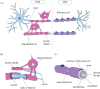
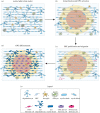
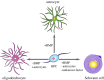
Myelin sheaths, by supporting axonal integrity and allowing rapid saltatory impulse conduction, are of fundamental importance for neuronal function. In response to demyelinating injuries in the central nervous system (CNS), oligodendrocyte progenitor cells (OPCs) migrate to the lesion area, proliferate and differentiate into new oligodendrocytes that make new myelin sheaths. This process is termed remyelination. Under specific conditions, demyelinated axons in the CNS can also be remyelinated by Schwann cells (SCs), the myelinating cell of the peripheral nervous system. OPCs can be a major source of these CNS-resident SCs-a surprising finding given the distinct embryonic origins, and physiological compartmentalization of the peripheral and central nervous system. Although the mechanisms and cues governing OPC-to-SC differentiation remain largely undiscovered, it might nevertheless be an attractive target for promoting endogenous remyelination. This article will (i) review current knowledge on the origins of SCs in the CNS, with a particular focus on OPC to SC differentiation, (ii) discuss the necessary criteria for SC myelination in the CNS and (iii) highlight the potential of using SCs for myelin regeneration in the CNS.
Keywords: Schwann cells; astrocytes; central nervous system; myelin; oligodendrocyte progenitor cells; remyelination.
Conflict of interest statement
We declare we have no competing interests.
Figures
Similar articles
-
A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System.J Neurosci. 2018 Oct 24;38(43):9228-9239. doi: 10.1523/JNEUROSCI.0585-18.2018. Epub 2018 Sep 18. J Neurosci. 2018. PMID: 30228229 Free PMC article.
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
-
Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS.Acta Neuropathol. 2012 Dec;124(6):847-60. doi: 10.1007/s00401-012-1034-0. Epub 2012 Aug 30. Acta Neuropathol. 2012. PMID: 22933014 Free PMC article.
-
Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination.Am J Pathol. 2015 Sep;185(9):2431-40. doi: 10.1016/j.ajpath.2015.05.011. Epub 2015 Jul 17. Am J Pathol. 2015. PMID: 26193667 Free PMC article.
-
Schwann cells: Rescuers of central demyelination.Glia. 2020 Oct;68(10):1945-1956. doi: 10.1002/glia.23788. Epub 2020 Feb 6. Glia. 2020. PMID: 32027054 Review.
Cited by
-
Melittin promotes the proliferation of Schwann cells in hyperglycemic environment by up‑regulating the Crabp2/Wnt/β‑catenin signaling pathway.Mol Med Rep. 2025 Jan;31(1):5. doi: 10.3892/mmr.2024.13371. Epub 2024 Oct 25. Mol Med Rep. 2025. PMID: 39450531 Free PMC article.
-
Exogenous Antioxidants in Remyelination and Skeletal Muscle Recovery.Biomedicines. 2022 Oct 13;10(10):2557. doi: 10.3390/biomedicines10102557. Biomedicines. 2022. PMID: 36289819 Free PMC article. Review.
-
Neuroplasticity and Nervous System Recovery: Cellular Mechanisms, Therapeutic Advances, and Future Prospects.Brain Sci. 2025 Apr 15;15(4):400. doi: 10.3390/brainsci15040400. Brain Sci. 2025. PMID: 40309875 Free PMC article. Review.
-
What Are the Roles of Oligodendrocyte Precursor Cells in Normal and Pathologic Conditions?Neurology. 2023 Nov 21;101(21):958-965. doi: 10.1212/WNL.0000000000208000. Neurology. 2023. PMID: 37985182 Free PMC article. No abstract available.
-
Perineural dexamethasone: neurotoxicity or neuroprotection? A systematic review of preclinical evidence.J Anesth Analg Crit Care. 2025 Aug 6;5(1):50. doi: 10.1186/s44158-025-00271-w. J Anesth Analg Crit Care. 2025. PMID: 40770367 Free PMC article. Review.
References
-
- Blakemore WF 1976. Invasion of Schwann cells into the spinal cord of the rat following local injections of lysolecithin. Neuropathol. Appl. Neurobiol. 2, 21–39. (10.1111/j.1365-2990.1976.tb00559.x) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

