Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology
- PMID: 33498273
- PMCID: PMC7909250
- DOI: 10.3390/cells10020199
Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology
Abstract
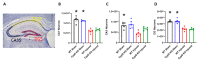
Cyclophilin D (CypD) has been shown to play a critical role in mitochondrial permeability transition pore (mPTP) opening and the subsequent cell death cascade. Studies consistently demonstrate that mitochondrial dysfunction, including mitochondrial calcium overload and mPTP opening, is essential to the pathobiology of cell death after a traumatic brain injury (TBI). CypD inhibitors, such as cyclosporin A (CsA) or NIM811, administered following TBI, are neuroprotective and quell neurological deficits. However, some pharmacological inhibitors of CypD have multiple biological targets and, as such, do not directly implicate a role for CypD in arbitrating cell death after TBI. Here, we reviewed the current understanding of the role CypD plays in TBI pathobiology. Further, we directly assessed the role of CypD in mediating cell death following TBI by utilizing mice lacking the CypD encoding gene Ppif. Following controlled cortical impact (CCI), the genetic knockout of CypD protected acute mitochondrial bioenergetics at 6 h post-injury and reduced subacute cortical tissue and hippocampal cell loss at 18 d post-injury. The administration of CsA following experimental TBI in Ppif-/- mice improved cortical tissue sparing, highlighting the multiple cellular targets of CsA in the mitigation of TBI pathology. The loss of CypD appeared to desensitize the mitochondrial response to calcium burden induced by TBI; this maintenance of mitochondrial function underlies the observed neuroprotective effect of the CypD knockout. These studies highlight the importance of maintaining mitochondrial homeostasis after injury and validate CypD as a therapeutic target for TBI. Further, these results solidify the beneficial effects of CsA treatment following TBI.
Keywords: NIM811; Ppif; controlled cortical impact; cyclosporin a; mitochondria; mitochondrial bioenergetics; mitochondrial permeability transition; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



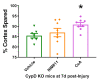
References
-
- Centers for Disease Control and Prevention TBI Data and Statistics. [(accessed on 26 October 2020)]; Available online: https://www.cdc.gov/traumaticbraininjury/data/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

