Iron Therapy in Chronic Kidney Disease: Days of Future Past
- PMID: 33498292
- PMCID: PMC7863960
- DOI: 10.3390/ijms22031008
Iron Therapy in Chronic Kidney Disease: Days of Future Past
Abstract
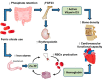
Anemia affects millions of patients with chronic kidney disease (CKD) and prompt iron supplementation can lead to reductions in the required dose of erythropoiesis-stimulating agents, thereby reducing medical costs. Oral and intravenous (IV) traditional iron preparations are considered far from ideal, primarily due to gastrointestinal intolerability and the potential risk of infusion reactions, respectively. Fortunately, the emergence of novel iron replacement therapies has engendered a paradigm shift in the treatment of iron deficiency anemia in patients with CKD. For example, oral ferric citrate is an efficacious and safe phosphate binder that increases iron stores to maintain hemoglobin levels. Additional benefits include reductions in fibroblast growth factor 23 levels and the activation of 1,25 dihydroxyvitamin D. The new-generation IV iron preparations ferumoxytol, iron isomaltoside 1000, and ferric carboxymaltose are characterized by a reduced risk of infusion reactions and are clinically well tolerated as a rapid high-dose infusion. In patients undergoing hemodialysis (HD), ferric pyrophosphate citrate (FPC) administered through dialysate enables the replacement of ongoing uremic and HD-related iron loss. FPC transports iron directly to transferrin, bypassing the reticuloendothelial system and avoiding iron sequestration. Moreover, this paper summarizes recent advancements of hypoxia-inducible factor prolyl hydroxylase inhibitors and future perspectives in renal anemia management.
Keywords: anemia; chronic kidney disease; ferric citrate; hypoxia-inducible factor; iron therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

