Neuroendocrine Neoplasms: Identification of Novel Metabolic Circuits of Potential Diagnostic Utility
- PMID: 33498434
- PMCID: PMC7864182
- DOI: 10.3390/cancers13030374
Neuroendocrine Neoplasms: Identification of Novel Metabolic Circuits of Potential Diagnostic Utility
Abstract
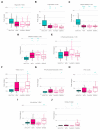
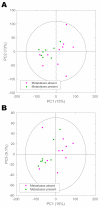
The incidence of neuroendocrine neoplasms (NEN) is increasing, but established biomarkers have poor diagnostic and prognostic accuracy. Here, we aim to define the systemic metabolic consequences of NEN and to establish the diagnostic utility of proton nuclear magnetic resonance spectroscopy (1H-NMR) for NEN in a prospective cohort of patients through a single-centre, prospective controlled observational study. Urine samples of 34 treatment-naïve NEN patients (median age: 59.3 years, range: 36-85): 18 had pancreatic (Pan) NEN, of which seven were functioning; 16 had small bowel (SB) NEN; 20 age- and sex-matched healthy control individuals were analysed using a 600 MHz Bruker 1H-NMR spectrometer. Orthogonal partial-least-squares-discriminant analysis models were able to discriminate both PanNEN and SBNEN patients from healthy control (Healthy vs. PanNEN: AUC = 0.90, Healthy vs. SBNEN: AUC = 0.90). Secondary metabolites of tryptophan, such as trigonelline and a niacin-related metabolite were also identified to be universally decreased in NEN patients, while upstream metabolites, such as kynurenine, were elevated in SBNEN. Hippurate, a gut-derived metabolite, was reduced in all patients, whereas other gut microbial co-metabolites, trimethylamine-N-oxide, 4-hydroxyphenylacetate and phenylacetylglutamine, were elevated in those with SBNEN. These findings suggest the existence of a new systems-based neuroendocrine circuit, regulated in part by cancer metabolism, neuroendocrine signalling molecules and gut microbial co-metabolism. Metabonomic profiling of NEN has diagnostic potential and could be used for discovering biomarkers for these tumours. These preliminary data require confirmation in a larger cohort.
Keywords: biomarkers; metabolic profiling; metabonomics; neuroendocrine neoplasms; neuroendocrine tumours; nuclear magnetic resonance; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Metabonomic profiling: a novel approach in neuroendocrine neoplasias.Surgery. 2013 Dec;154(6):1185-92; discussion 1192-3. doi: 10.1016/j.surg.2013.06.018. Surgery. 2013. PMID: 24383116
-
Race and Odds of Surgery Offer in Small Bowel and Pancreas Neuroendocrine Neoplasms.Ann Surg Oncol. 2024 May;31(5):3249-3260. doi: 10.1245/s10434-024-14906-9. Epub 2024 Jan 31. Ann Surg Oncol. 2024. PMID: 38294612
-
Metabolic signatures of lung cancer in biofluids: NMR-based metabonomics of urine.J Proteome Res. 2011 Jan 7;10(1):221-30. doi: 10.1021/pr100899x. Epub 2010 Nov 23. J Proteome Res. 2011. PMID: 21058631
-
Decoding the Molecular and Mutational Ambiguities of Gastroenteropancreatic Neuroendocrine Neoplasm Pathobiology.Cell Mol Gastroenterol Hepatol. 2015 Jan 12;1(2):131-153. doi: 10.1016/j.jcmgh.2014.12.008. eCollection 2015 Mar. Cell Mol Gastroenterol Hepatol. 2015. PMID: 28210673 Free PMC article. Review.
-
Neuroendocrine neoplasms of the gastroenteropancreatic system: pathology and classification.Horm Metab Res. 2011 Nov;43(12):825-31. doi: 10.1055/s-0031-1291307. Epub 2011 Nov 21. Horm Metab Res. 2011. PMID: 22105474 Review.
Cited by
-
An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms.World J Gastroenterol. 2022 Mar 14;28(10):1009-1023. doi: 10.3748/wjg.v28.i10.1009. World J Gastroenterol. 2022. PMID: 35431496 Free PMC article. Review.
-
FOXA2-initiated transcriptional activation of INHBA induced by methylmalonic acid promotes pancreatic neuroendocrine neoplasm progression.Cell Mol Life Sci. 2024 Jan 22;81(1):50. doi: 10.1007/s00018-023-05084-0. Cell Mol Life Sci. 2024. PMID: 38252148 Free PMC article.
-
Metabolic changes in neuroendocrine neoplasms.Cell Mol Life Sci. 2025 May 16;82(1):205. doi: 10.1007/s00018-025-05656-2. Cell Mol Life Sci. 2025. PMID: 40377669 Free PMC article. Review.
References
-
- Yao J.C., Guthrie K.A., Moran C., Strosberg J.R., Kulke M.H., Chan J.A., LoConte N., McWilliams R.R., Wolin E.M., Mattar B., et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients with Advanced Carcinoid Tumors: SWOG S0518. J. Clin. Oncol. 2017;35:1695–1703. doi: 10.1200/JCO.2016.70.4072. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

