Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020
- PMID: 33498438
- PMCID: PMC7909408
- DOI: 10.3390/toxins13020075
Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020
Abstract
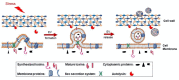
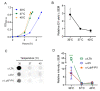
Staphylococcus aureus generates and releases extracellular vesicles (EVs) that package cytosolic, cell-wall associated, and membrane proteins, as well as glycopolymers and exoproteins, including alpha hemolysin, leukocidins, phenol-soluble modulins, superantigens, and enzymes. S. aureus EVs, but not EVs from pore-forming toxin-deficient strains, were cytolytic for a variety of mammalian cell types, but EV internalization was not essential for cytotoxicity. Because S. aureus is subject to various environmental stresses during its encounters with the host during infection, we assessed how these exposures affected EV production in vitro. Staphylococci grown at 37 °C or 40 °C did not differ in EV production, but cultures incubated at 30 °C yielded more EVs when grown to the same optical density.S. aureus cultivated in the presence of oxidative stress, in iron-limited media, or with subinhibitory concentrations of ethanol, showed greater EV production as determined by protein yield and quantitative immunoblots. In contrast, hyperosmotic stress or subinhibitory concentrations of erythromycin reduced S. aureus EV yield. EVs represent a novel S. aureus secretory system that is affected by a variety of stress responses and allows the delivery of biologically active pore-forming toxins and other virulence determinants to host cells.
Keywords: Staphylococcus aureus; extracellular vesicles; stress; toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



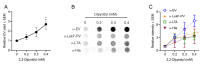


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

