A Two-Scale Multi-Resolution Topologically Optimized Multi-Material Design of 3D Printed Craniofacial Bone Implants
- PMID: 33498498
- PMCID: PMC7909579
- DOI: 10.3390/mi12020101
A Two-Scale Multi-Resolution Topologically Optimized Multi-Material Design of 3D Printed Craniofacial Bone Implants
Abstract
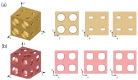
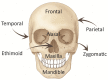
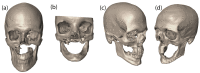
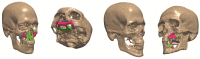
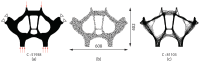
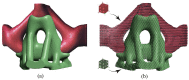
Bone replacement implants for craniofacial reconstruction require to provide an adequate structural foundation to withstand the physiological loading. With recent advances in 3D printing technology in place of bone grafts using autologous tissues, patient-specific additively manufactured implants are being established as suitable alternates. Since the stress distribution of these structures is complicated, efficient design techniques, such as topology optimization, can deliver optimized designs with enhanced functionality. In this work, a two-scale topology optimization approach is proposed that provides multi-material designs for both macrostructures and microstructures. In the first stage, a multi-resolution topology optimization approach is used to produce multi-material designs with maximum stiffness. Then, a microstructure with a desired property supplants the solid domain. This is beneficial for bone implant design since, in addition to imparting the desired functional property to the design, it also introduces porosity. To show the efficacy of the technique, four different large craniofacial defects due to maxillectomy are considered, and their respective implant designs with multi-materials are shown. These designs show good potential in developing patient-specific optimized designs suitable for additive manufacturing.
Keywords: bone implants; bone replacements; craniofacial surgery; multi-material; topology optimization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Buckwalter J.A., Cooper R.R. Bone structure and function. Instr. Course Lect. 1987;36:27–48. - PubMed
-
- Weiner S., Wagner H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998;28:271–298. doi: 10.1146/annurev.matsci.28.1.271. - DOI
-
- Oldani C., Dominguez A. Titanium as a Biomaterial for Implants. Recent Adv. Arthroplast. 2012;218:149–162.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

