Urban Aerosol Particulate Matter Promotes Necrosis and Autophagy via Reactive Oxygen Species-Mediated Cellular Disorders that are Accompanied by Cell Cycle Arrest in Retinal Pigment Epithelial Cells
- PMID: 33498524
- PMCID: PMC7909535
- DOI: 10.3390/antiox10020149
Urban Aerosol Particulate Matter Promotes Necrosis and Autophagy via Reactive Oxygen Species-Mediated Cellular Disorders that are Accompanied by Cell Cycle Arrest in Retinal Pigment Epithelial Cells
Abstract
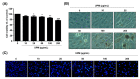
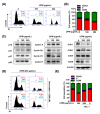
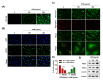
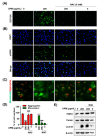
Urban particulate matter (UPM) is recognized as a grave public health problem worldwide. Although a few studies have linked UPM to ocular surface diseases, few studies have reported on retinal dysfunction. Thus, the aim of the present study was to evaluate the influence of UPM on the retina and identify the main mechanism of UPM toxicity. In this study, we found that UPM significantly induced cytotoxicity with morphological changes in ARPE-19 human retinal pigment epithelial (RPE) cells and increased necrosis and autophagy but not apoptosis. Furthermore, UPM significantly increased G2/M arrest and simultaneously induced alterations in cell cycle regulators. In addition, DNA damage and mitochondrial dysfunction were remarkably enhanced by UPM. However, the pretreatment with the potent reactive oxygen species (ROS) scavenger N-acetyl-L-cysteine (NAC) effectively suppressed UPM-mediated cytotoxicity, necrosis, autophagy, and cell cycle arrest. Moreover, NAC markedly restored UPM-induced DNA damage and mitochondrial dysfunction. Meanwhile, UPM increased the expression of mitophagy-regulated proteins, but NAC had no effect on mitophagy. Taken together, although further studies are needed to identify the role of mitophagy in UPM-induced RPE injury, the present study provides the first evidence that ROS-mediated cellular damage through necrosis and autophagy is one of the mechanisms of UPM-induced retinal disorders.
Keywords: mitophagy; necrosis; reactive oxygen species (ROS); retinal pigment epithelial (RPE) cells; urban aerosol particulate matter (UPM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Urban aerosol particulate matter promotes cellular senescence through mitochondrial ROS-mediated Akt/Nrf2 downregulation in human retinal pigment epithelial cells.Free Radic Res. 2024 Dec;58(12):841-853. doi: 10.1080/10715762.2024.2438919. Epub 2024 Dec 8. Free Radic Res. 2024. PMID: 39645666
-
Urban aerosol particulate matter promotes mitochondrial oxidative stress-induced cellular senescence in human retinal pigment epithelial ARPE-19 cells.Environ Toxicol Pharmacol. 2023 Sep;102:104211. doi: 10.1016/j.etap.2023.104211. Epub 2023 Jul 7. Environ Toxicol Pharmacol. 2023. PMID: 37423393
-
Diesel particulate matter2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species.Environ Pollut. 2020 Jul;262:114301. doi: 10.1016/j.envpol.2020.114301. Epub 2020 Mar 4. Environ Pollut. 2020. PMID: 32155554
-
Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death.J Biomed Sci. 2019 May 22;26(1):40. doi: 10.1186/s12929-019-0531-z. J Biomed Sci. 2019. PMID: 31118030 Free PMC article.
-
Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells.Toxicol Lett. 2019 Sep 1;311:105-113. doi: 10.1016/j.toxlet.2019.05.001. Epub 2019 May 2. Toxicol Lett. 2019. PMID: 31054874
Cited by
-
The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5.Nutrients. 2021 Aug 27;13(9):2986. doi: 10.3390/nu13092986. Nutrients. 2021. PMID: 34578864 Free PMC article.
-
The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5.Pharmaceutics. 2021 Sep 10;13(9):1439. doi: 10.3390/pharmaceutics13091439. Pharmaceutics. 2021. PMID: 34575516 Free PMC article.
-
Nrf2-mediated activation of HO-1 is required in the blocking effect of compound K, a ginseng saponin metabolite, against oxidative stress damage in ARPE-19 human retinal pigment epithelial cells.J Ginseng Res. 2023 Mar;47(2):311-318. doi: 10.1016/j.jgr.2022.09.007. Epub 2022 Oct 5. J Ginseng Res. 2023. PMID: 36926611 Free PMC article.
-
Fisetin Attenuated Oxidative Stress-Induced Cellular Damage in ARPE-19 Human Retinal Pigment Epithelial Cells Through Nrf2-Mediated Activation of Heme Oxygenase-1.Front Pharmacol. 2022 Jun 16;13:927898. doi: 10.3389/fphar.2022.927898. eCollection 2022. Front Pharmacol. 2022. PMID: 35784747 Free PMC article.
-
Pathological Mechanisms of Particulate Matter-Mediated Ocular Disorders: A Review.Int J Mol Sci. 2024 Nov 11;25(22):12107. doi: 10.3390/ijms252212107. Int J Mol Sci. 2024. PMID: 39596177 Free PMC article. Review.
References
-
- Bakolis I., Hammoud R., Stewart R., Beevers S., Dajnak D., MacCrimmon S., Broadbent M., Pritchard M., Shiode N., Fecht D., et al. Mental health consequences of urban air pollution: Prospective population-based longitudinal survey. Soc. Psychiatry Psychiatr. Epidemiol. 2020:1–13. doi: 10.1007/s00127-020-01966-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

