NTPDase1 Modulates Smooth Muscle Contraction in Mice Bladder by Regulating Nucleotide Receptor Activation Distinctly in Male and Female
- PMID: 33498759
- PMCID: PMC7911947
- DOI: 10.3390/biom11020147
NTPDase1 Modulates Smooth Muscle Contraction in Mice Bladder by Regulating Nucleotide Receptor Activation Distinctly in Male and Female
Abstract
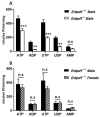
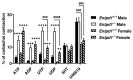
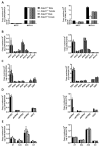
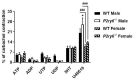
Nucleotides released by smooth muscle cells (SMCs) and by innervating nerve terminals activate specific P2 receptors and modulate bladder contraction. We hypothesized that cell surface enzymes regulate SMC contraction in mice bladder by controlling the concentration of nucleotides. We showed by immunohistochemistry, enzymatic histochemistry, and biochemical activities that nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) and ecto-5'-nucleotidase were the major ectonucleotidases expressed by SMCs in the bladder. RT-qPCR revealed that, among the nucleotide receptors, there was higher expression of P2X1, P2Y1, and P2Y6 receptors. Ex vivo, nucleotides induced a more potent contraction of bladder strips isolated from NTPDase1 deficient (Entpd1-/-) mice compared to wild type controls. The strongest responses were obtained with uridine 5'-triphosphate (UTP) and uridine 5'-diphosphate (UDP), suggesting the involvement of P2Y6 receptors, which was confirmed with P2ry6-/- bladder strips. Interestingly, this response was reduced in female bladders. Our results also suggest the participation of P2X1, P2Y2 and/or P2Y4, and P2Y12 in these contractions. A reduced response to the thromboxane analogue U46619 was also observed in wild type, Entpd1-/-, and P2ry6-/- female bladders showing another difference due to sex. In summary, NTPDase1 modulates the activation of nucleotide receptors in mouse bladder SMCs, and contractions induced by P2Y6 receptor activation were weaker in female bladders.
Keywords: NTPDase1; P2Y6 receptor; bladder; contraction; extracellular nucleotides; sex; smooth muscle cells.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures




References
-
- Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C., Knight G.E., Fumagalli M., Gachet C., Jacobson K.A., et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

