The Regulation of Nodule Number in Legumes Is a Balance of Three Signal Transduction Pathways
- PMID: 33498783
- PMCID: PMC7866212
- DOI: 10.3390/ijms22031117
The Regulation of Nodule Number in Legumes Is a Balance of Three Signal Transduction Pathways
Abstract
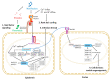
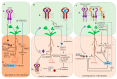
Nitrogen is a major determinant of plant growth and productivity and the ability of legumes to form a symbiotic relationship with nitrogen-fixing rhizobia bacteria allows legumes to exploit nitrogen-poor niches in the biosphere. But hosting nitrogen-fixing bacteria comes with a metabolic cost, and the process requires regulation. The symbiosis is regulated through three signal transduction pathways: in response to available nitrogen, at the initiation of contact between the organisms, and during the development of the nodules that will host the rhizobia. Here we provide an overview of our knowledge of how the three signaling pathways operate in space and time, and what we know about the cross-talk between symbiotic signaling for nodule initiation and organogenesis, nitrate dependent signaling, and autoregulation of nodulation. Identification of common components and points of intersection suggest directions for research on the fine-tuning of the plant's response to rhizobia.
Keywords: Medicago truncatula; autoregulation of nodulation; nitrogen response in nodulation; nodulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Herridge D.F., Peoples M.B., Boddey R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil. 2008;311:1–18. doi: 10.1007/s11104-008-9668-3. - DOI
-
- Ruschel A.P., Vose P., Victoria R., Salati E. Comparison of Isotope Techniques and Non-Nodulating Isolines to Study the Effect of Ammonium Fertilization on Dinitrogen Fixation in Soybean, Glycine max. Plant Soil. 1979;53:513–525. doi: 10.1007/BF02140722. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

