Dissociation Constant of Integrin-RGD Binding in Live Cells from Automated Micropipette and Label-Free Optical Data
- PMID: 33498959
- PMCID: PMC7911545
- DOI: 10.3390/bios11020032
Dissociation Constant of Integrin-RGD Binding in Live Cells from Automated Micropipette and Label-Free Optical Data
Abstract
The binding of integrin proteins to peptide sequences such as arginine-glycine-aspartic acid (RGD) is a crucial step in the adhesion process of mammalian cells. While these bonds can be examined between purified proteins and their ligands, live-cell assays are better suited to gain biologically relevant information. Here we apply a computer-controlled micropipette (CCMP) to measure the dissociation constant (Kd) of integrin-RGD-binding. Surface coatings with varying RGD densities were prepared, and the detachment of single cells from these surfaces was measured by applying a local flow inducing hydrodynamic lifting force on the targeted cells in discrete steps. The average behavior of the populations was then fit according to the chemical law of mass action. To verify the resulting value of Kd2d = (4503 ± 1673) 1/µm2, a resonant waveguide grating based biosensor was used, characterizing and fitting the adhesion kinetics of the cell populations. Both methods yielded a Kd within the same range. Furthermore, an analysis of subpopulations was presented, confirming the ability of CCMP to characterize cell adhesion both on single cell and whole population levels. The introduced methodologies offer convenient and automated routes to quantify the adhesivity of living cells before their further processing.
Keywords: adhesion; biosensor; integrin-RGD-binding; micropipette; two-dimensional dissociation constant; waveguide.
Conflict of interest statement
Balint Szabo is the founder of the CellSorter Biotechnology Innovations Company.
Figures

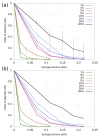

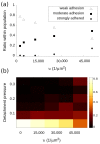
References
-
- Beckerle M.C. Cell Adhesion. Oxford University Press Inc.; New York, NY, USA: 2001.
-
- Slack J.M.W. Principles of Tissue Engineering. 4th ed. Elsevier; Amsterdam, The Netherlands: 2013. Molecular Biology of the Cell. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

