Evidence for Establishing the Clinical Breakpoint of Cefquinome against Haemophilus Parasuis in China
- PMID: 33498972
- PMCID: PMC7912692
- DOI: 10.3390/pathogens10020105
Evidence for Establishing the Clinical Breakpoint of Cefquinome against Haemophilus Parasuis in China
Abstract
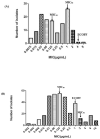
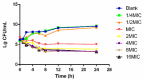
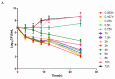
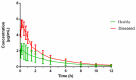
Haemophilus parasuis can cause high morbidity and mortality in swine. Cefquinome possesses excellent antibacterial activity against pathogens causing diseases of the respiratory tract. This study aimed to establish the clinical breakpoint (CBP) of cefquinome against H. parasuis and to monitor the resistance change. Referring to the minimum inhibitory concentration (MIC) distribution of cefquinome against 131 H. parasuis isolates, the MIC50 and MIC90 were determined to be 0.125 and 1 μg/mL, respectively. And the epidemiological cutoff (ECOFF) value was 1 μg/mL. HPS42 was selected as a representative strain for the pharmacodynamic (PD) experiment, pharmacokinetic (PK) experiment and clinical experiments. The PK/PD index values, area under concentration-time curve (AUC)/MIC, of the bacteriostatic, bactericidal, and bacterial elimination effects were 23, 41, and 51 h, respectively. The PK/PD cutoff was calculated as 0.125 μg/mL by Monte Carlo simulation (MCS), and the clinical cutoff was 0.25-4 μg/mL by WindoW. Combing these three values, the CBP of cefquinome against H. parasuis was found to be 1 μg/mL. In conclusion, this was the first study to integrate various cutoffs to establish the CBP in the laboratory. It is helpful to distinguish wild type H. parasuis and reduce the probability of treatment failure.
Keywords: Haemophilus parasuis; PK/PD cutoff; cefquinome; clinical breakpoint; clinical cutoff; epidemiological cutoff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Toutain P., Bousquet-Mélou A., Damborg P., Ferran A.A., Mevius D., Pelligand L., Veldman K.T., Lees P. En Route towards European Clinical Breakpoints for Veterinary Antimicrobial Susceptibility Testing: A Position Paper Explaining the VetCAST Approach. Front. Microbiol. 2017;8:2344. doi: 10.3389/fmicb.2017.02344. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

