Liraglutide-Induced Hepatotoxicity
- PMID: 33498980
- PMCID: PMC7911918
- DOI: 10.3390/biomedicines9020106
Liraglutide-Induced Hepatotoxicity
Abstract
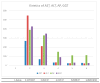
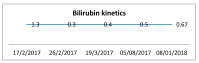
A 52-year-old woman with a BMI of 31.2 kg/m2 was treated with the glucagon-like peptide 1 (GLP-1) agonist liraglutide as part of her weight-reduction program. Following this, she developed an idiosyncratic drug-related liver injury (IDILI). Advances in noninvasive techniques enabled this diagnosis to be established. By employing easily quantifiable methods based on serum biomarkers, we could explore a wide variety of endpoints in assessing personalized DILI. In addition, we can test endpoints that are associated with the drug's mechanism of action. Personalized medicine and therapeutic pharmacovigilance of incretin-based hypoglycemic agents are needed to ensure the safety of patients.
Keywords: drug-induced liver injury; liraglutide-induced immune hepatitis; lymphocyte toxicity assay; personalized medicine.
Conflict of interest statement
The authors declare no conflict of interest. All the authors had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All the authors contributed to the study concept and design: acquisition, analysis and interpretation of data and drafting of the manuscript and revision of the manuscript for intellectual content. Technical support was provided by In Vitro Drug Safety and Biotechnology.
Figures
References
-
- [(accessed on 19 December 2020)]; Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-an....
-
- [(accessed on 6 April 2016)]; Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-an....
-
- Watada H., Kaneko S., Komatsu M., Agner B.R., Nishida T., Ranthe M., Nakamura J. Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with ≤50 units insulin degludec in Japanese subjects with type 2 diabetes in a phase 3, double-blind, randomised trial. Diabetes Obes. Metab. 2019;21:2694–2703. doi: 10.1111/dom.13859. - DOI - PMC - PubMed
-
- Nauck M.A., Ghorbani M.L.M., Kreiner E., Saevereid H.A., Buse J.B. LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effects of Liraglutide Compared with Placebo on Events of Acute Gallbladder or Biliary Disease in Patients with Type 2 Diabetes at High Risk for Cardiovascular Events in the LEADER Randomized Trial. Diabetes Care. 2019;42:1912–1920. doi: 10.2337/dc19-0415. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources