The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond
- PMID: 33499062
- PMCID: PMC7912299
- DOI: 10.3390/biom11020141
The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond
Abstract
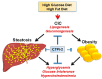
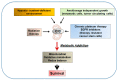
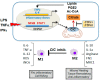
The mitochondrial citrate/isocitrate carrier, CIC, has been shown to play an important role in a growing list of human diseases. CIC belongs to a large family of nuclear-encoded mitochondrial transporters that serve the fundamental function of allowing the transit of ions and metabolites through the impermeable mitochondrial membrane. Citrate is central to mitochondrial metabolism and respiration and plays fundamental activities in the cytosol, serving as a metabolic substrate, an allosteric enzymatic regulator and, as the source of Acetyl-Coenzyme A, also as an epigenetic modifier. In this review, we highlight the complexity of the mechanisms of action of this transporter, describing its involvement in human diseases and the therapeutic opportunities for targeting its activity in several pathological conditions.
Keywords: 22.q11.2; CIC; CTP; NAFLD/NASH; SLC25A1; cancer; citrate; diabetes; inflammation; metabolism; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

