Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH
- PMID: 33499064
- PMCID: PMC7865890
- DOI: 10.3390/polym13030348
Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH
Abstract
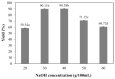
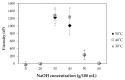
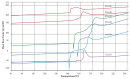
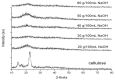
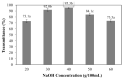
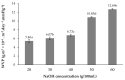
Bacterial cellulose from nata de coco was prepared from the fermentation of coconut juice with Acetobacter xylinum for 10 days at room temperature under sterile conditions. Carboxymethyl cellulose (CMC) was transformed from the bacterial cellulose from the nata de coco by carboxymethylation using different concentrations of sodium hydroxide (NaOH) and monochloroacetic acid (MCA) in an isopropyl (IPA) medium. The effects of various NaOH concentrations on the degree of substitution (DS), chemical structure, viscosity, color, crystallinity, morphology and the thermal properties of carboxymethyl bacterial cellulose powder from nata de coco (CMCn) were evaluated. In the carboxymethylation process, the optimal condition resulted from NaOH amount of 30 g/100 mL, as this provided the highest DS value (0.92). The crystallinity of CMCn declined after synthesis but seemed to be the same in each condition. The mechanical properties (tensile strength and percentage of elongation at break), water vapor permeability (WVP) and morphology of CMCn films obtained from CMCn synthesis using different NaOH concentrations were investigated. The tensile strength of CMCn film synthesized with a NaOH concentration of 30 g/100 mL increased, however it declined when the amount of NaOH concentration was too high. This result correlated with the DS value. The highest percent elongation at break was obtained from CMCn films synthesized with 50 g/100 mL NaOH, whereas the elongation at break decreased when NaOH concentration increased to 60 g/100 mL.
Keywords: CMC; bacterial cellulose; biopolymer; carboxymethyl cellulose; nata de coco; sodium hydroxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Crawford D.L., Barder M.J., Pometto A.L., Crawford R.L. Chemistry of softwood lignin degradation by Strepto-myces viridosporus. Arch. Microbiol. 1982;131:140–145. doi: 10.1007/BF01053996. - DOI
-
- Rachtanapun P., Luangkamin S., Tanprasert K., Suriyatem R. Carboxymethyl cellulose film from durian rind. LWT-Food Sci. Technol. 2012;48:52–58. doi: 10.1016/j.lwt.2012.02.029. - DOI
-
- Rachtanapun P., Eitssayeam S., Pengpat K. Study of Carboxymethyl Cellulose from Papaya Peels as Binder in Ceramics. Adv. Mater. Res. 2010;93–94:17–21. doi: 10.4028/www.scientific.net/AMR.93-94.17. - DOI
-
- Sun R., Hughes S. Fractional extraction and physico-chemical characterization of hemicelluloses and cellulose from sugar beet pulp. Carbohydr. Polym. 1998;36:293–299. doi: 10.1016/S0144-8617(97)00255-5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

