Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours
- PMID: 33499110
- PMCID: PMC7865297
- DOI: 10.3390/ijms22031076
Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours
Abstract
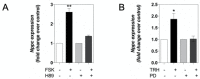
Patients harbouring mutations in genes encoding C-type natriuretic peptide (CNP; NPPC) or its receptor guanylyl cyclase B (GC-B, NPR2) suffer from severe growth phenotypes; loss-of-function mutations cause achondroplasia, whereas gain-of-function mutations cause skeletal overgrowth. Although most of the effects of CNP/GC-B on growth are mediated directly on bone, evidence suggests the natriuretic peptides may also affect anterior pituitary control of growth. Our previous studies described the expression of NPPC and NPR2 in a range of human pituitary tumours, normal human pituitary, and normal fetal human pituitary. However, the natriuretic peptide system in somatotropes has not been extensively explored. Here, we examine the expression and function of the CNP/GC-B system in rat GH3 somatolactotrope cell line and pituitary tumours from a cohort of feline hypersomatotropism (HST; acromegaly) patients. Using multiplex RT-qPCR, all three natriuretic peptides and their receptors were detected in GH3 cells. The expression of Nppc was significantly enhanced following treatment with either 100 nM TRH or 10 µM forskolin, yet only Npr1 expression was sensitive to forskolin stimulation; the effects of forskolin and TRH on Nppc expression were PKA- and MAPK-dependent, respectively. CNP stimulation of GH3 somatolactotropes significantly inhibited Esr1, Insr and Lepr expression, but dramatically enhanced cFos expression at the same time point. Oestrogen treatment significantly enhanced expression of Nppa, Nppc, Npr1, and Npr2 in GH3 somatolactotropes, but inhibited CNP-stimulated cGMP accumulation. Finally, transcripts for all three natriuretic peptides and receptors were expressed in feline pituitary tumours from patients with HST. NPPC expression was negatively correlated with pituitary tumour volume and SSTR5 expression, but positively correlated with D2R and GHR expression. Collectively, these data provide mechanisms that control expression and function of CNP in somatolactotrope cells, and identify putative transcriptional targets for CNP action in somatotropes.
Keywords: CNP; acromegaly; feline; multiplex RT-qPCR; pituitary; somatotrope.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Homologous and heterologous desensitization of guanylyl cyclase-B signaling in GH3 somatolactotropes.Cell Tissue Res. 2014 Feb;355(2):425-36. doi: 10.1007/s00441-013-1763-y. Epub 2013 Dec 20. Cell Tissue Res. 2014. PMID: 24352806 Free PMC article.
-
Molecular characterisation and functional interrogation of a local natriuretic peptide system in rodent pituitaries, alphaT3-1 and LbetaT2 gonadotroph cells.J Endocrinol. 2009 Nov;203(2):215-29. doi: 10.1677/JOE-09-0189. Epub 2009 Aug 7. J Endocrinol. 2009. PMID: 19666697
-
Expression of guanylyl cyclase-B (GC-B/NPR2) receptors in normal human fetal pituitaries and human pituitary adenomas implicates a role for C-type natriuretic peptide.Endocr Relat Cancer. 2012 Jul 22;19(4):497-508. doi: 10.1530/ERC-12-0129. Print 2012 Aug. Endocr Relat Cancer. 2012. PMID: 22645228
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
-
Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review.
Cited by
-
TRH Regulates the Synthesis and Secretion of Prolactin in Rats with Adenohypophysis through the Differential Expression of miR-126a-5p.Int J Mol Sci. 2022 Dec 14;23(24):15914. doi: 10.3390/ijms232415914. Int J Mol Sci. 2022. PMID: 36555554 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

