Lack of Electron Acceptors Contributes to Redox Stress and Growth Arrest in Asparagine-Starved Sarcoma Cells
- PMID: 33499165
- PMCID: PMC7865502
- DOI: 10.3390/cancers13030412
Lack of Electron Acceptors Contributes to Redox Stress and Growth Arrest in Asparagine-Starved Sarcoma Cells
Abstract
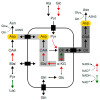
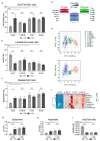
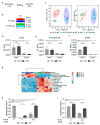
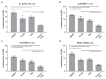
Amino acids are integral components of cancer metabolism. The non-essential amino acid asparagine supports the growth and survival of various cancer cell types. Here, different mass spectrometry approaches were employed to identify lower aspartate levels, higher aspartate/glutamine ratios and lower tricarboxylic acid (TCA) cycle metabolite levels in asparagine-deprived sarcoma cells. Reduced nicotinamide adenine dinucleotide (NAD+)/nicotinamide adenine dinucleotide hydride (NADH) ratios were consistent with redirection of TCA cycle flux and relative electron acceptor deficiency. Elevated lactate/pyruvate ratios may be due to compensatory NAD+ regeneration through increased pyruvate to lactate conversion by lactate dehydrogenase. Supplementation with exogenous pyruvate, which serves as an electron acceptor, restored aspartate levels, NAD+/NADH ratios, lactate/pyruvate ratios and cell growth in asparagine-deprived cells. Chemicals disrupting NAD+ regeneration in the electron transport chain further enhanced the anti-proliferative and pro-apoptotic effects of asparagine depletion. We speculate that reductive stress may be a major contributor to the growth arrest observed in asparagine-starved cells.
Keywords: asparagine starvation; metabolomics; reductive stress; sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. - DOI - PMC - PubMed
-
- Gwinn D.M., Lee A.G., Briones-Martin-Del-Campo M., Conn C.S., Simpson D.R., Scott A.I., Le A., Cowan T.M., Ruggero D., Sweet-Cordero E.A. Oncogenic KRAS Regulates Amino Acid Homeostasis and Asparagine Biosynthesis via ATF4 and Alters Sensitivity to L-Asparaginase. Cancer Cell. 2018;33:91–107. doi: 10.1016/j.ccell.2017.12.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

