Primary Pulmonary B-Cell Lymphoma: A Review and Update
- PMID: 33499258
- PMCID: PMC7865219
- DOI: 10.3390/cancers13030415
Primary Pulmonary B-Cell Lymphoma: A Review and Update
Abstract
Primary pulmonary B-cell lymphomas (PP-BCLs) comprise a group of extranodal non-Hodgkin lymphomas of B-cell origin, which primarily affect the lung without evidence of extrapulmonary disease at the time of diagnosis and up to 3 months afterwards. Primary lymphoid proliferations of the lung are most often of B-cell lineage, and include three major entities with different clinical, morphological, and molecular features: primary pulmonary marginal zone lymphoma of mucosa-associated lymphoid tissue (PP-MZL, or MALT lymphoma), primary pulmonary diffuse large B cell lymphoma (PP-DLBCL), and lymphomatoid granulomatosis (LYG). Less common entities include primary effusion B-cell lymphoma (PEL) and intravascular large B cell lymphoma (IVLBCL). A proper workup requires a multidisciplinary approach, including radiologists, pneumologists, thoracic surgeons, pathologists, hemato-oncologists, and radiation oncologists, in order to achieve a correct diagnosis and risk assessment. Aim of this review is to analyze and outline the clinical and pathological features of the most frequent PP-BCLs, and to critically analyze the major issues in their diagnosis and management.
Keywords: BALT; MALT lymphoma; diffuse large B-cell lymphoma; intravascular large B-cell lymphoma; lymphomatoid granulomatosis; primary effusion lymphoma; pulmonary B-cell lymphoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
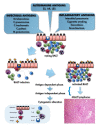


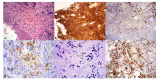
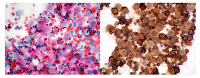

References
-
- Swerdlow S., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Arber D.A., Hasserjian R.P., Le Beau M.M., et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC; Lyon, France: 2017.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

