Optimising Vaccine Dose in Inoculation against SARS-CoV-2, a Multi-Factor Optimisation Modelling Study to Maximise Vaccine Safety and Efficacy
- PMID: 33499326
- PMCID: PMC7911627
- DOI: 10.3390/vaccines9020078
Optimising Vaccine Dose in Inoculation against SARS-CoV-2, a Multi-Factor Optimisation Modelling Study to Maximise Vaccine Safety and Efficacy
Abstract
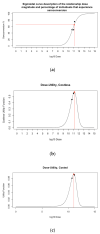
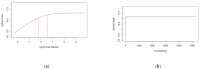
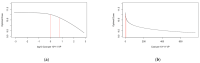
Developing a vaccine against the global pandemic SARS-CoV-2 is a critical area of active research. Modelling can be used to identify optimal vaccine dosing; maximising vaccine efficacy and safety and minimising cost. We calibrated statistical models to published dose-dependent seroconversion and adverse event data of a recombinant adenovirus type-5 (Ad5) SARS-CoV-2 vaccine given at doses 5.0 × 1010, 1.0 × 1011 and 1.5 × 1011 viral particles. We estimated the optimal dose for three objectives, finding: (A) the minimum dose that may induce herd immunity, (B) the dose that maximises immunogenicity and safety and (C) the dose that maximises immunogenicity and safety whilst minimising cost. Results suggest optimal dose [95% confidence interval] in viral particles per person was (A) 1.3 × 1011 [0.8-7.9 × 1011], (B) 1.5 × 1011 [0.3-5.0 × 1011] and (C) 1.1 × 1011 [0.2-1.5 × 1011]. Optimal dose exceeded 5.0 × 1010 viral particles only if the cost of delivery exceeded £0.65 or cost per 1011 viral particles was less than £6.23. Optimal dose may differ depending on the objectives of developers and policy-makers, but further research is required to improve the accuracy of optimal-dose estimates.
Keywords: COVID-19; adenovirus-vectored vaccines; dose dynamics; dose-response; dosing.
Conflict of interest statement
This work is partially funded by Vaccitech, a company that is developing novel adenoviral vector vaccines using the vectors ChAdOx1 and ChAdOx2.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

