Myocardial Blood Flow Control by Oxygen Sensing Vascular Kvβ Proteins
- PMID: 33499656
- PMCID: PMC8486354
- DOI: 10.1161/CIRCRESAHA.120.317715
Myocardial Blood Flow Control by Oxygen Sensing Vascular Kvβ Proteins
Abstract
[Figure: see text].
Keywords: aldo-keto reductases; coronary circulation; ion channels; physiology; potassium channels.
Figures




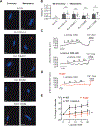
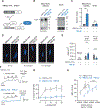
Comment in
-
Another Piece of the Puzzle: Voltage-Gated K+ Channel β2-Subunits as a Coronary Vascular Smooth Muscle Sensor of Cardiac Work.Circ Res. 2021 Mar 19;128(6):752-754. doi: 10.1161/CIRCRESAHA.121.318953. Epub 2021 Mar 18. Circ Res. 2021. PMID: 33734818 Free PMC article. No abstract available.
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. 2020:CIR0000000000000757 - PubMed
-
- Tsagalou EP, Anastasiou-Nana M, Agapitos E, Gika A, Drakos SG, Terrovitis JV, Ntalianis A, Nanas JN. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology. 2008;52:1391–1398 - PubMed
-
- Brush JE Jr., Cannon RO 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. The New England journal of medicine. 1988;319:1302–1307 - PubMed
-
- Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52:1369–1377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

