Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage
- PMID: 33499933
- PMCID: PMC7803124
- DOI: 10.1186/s42523-019-0004-4
Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage
Abstract
Background: Recent studies using batch-fermentation suggest that the red macroalgae Asparagopsis taxiformis has the potential to reduce methane (CH4) production from beef cattle by up to ~ 99% when added to Rhodes grass hay; a common feed in the Australian beef industry. These experiments have shown significant reductions in CH4 without compromising other fermentation parameters (i.e. volatile fatty acid production) with A. taxiformis organic matter (OM) inclusion rates of up to 5%. In the study presented here, A. taxiformis was evaluated for its ability to reduce methane production from dairy cattle fed a mixed ration widely utilized in California, the largest milk producing state in the US.
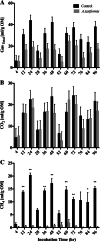
Results: Fermentation in a semi-continuous in-vitro rumen system suggests that A. taxiformis can reduce methane production from enteric fermentation in dairy cattle by 95% when added at a 5% OM inclusion rate without any obvious negative impacts on volatile fatty acid production. High-throughput 16S ribosomal RNA (rRNA) gene amplicon sequencing showed that seaweed amendment effects rumen microbiome consistent with the Anna Karenina hypothesis, with increased β-diversity, over time scales of approximately 3 days. The relative abundance of methanogens in the fermentation vessels amended with A. taxiformis decreased significantly compared to control vessels, but this reduction in methanogen abundance was only significant when averaged over the course of the experiment. Alternatively, significant reductions of CH4 in the A. taxiformis amended vessels was measured in the early stages of the experiment. This suggests that A. taxiformis has an immediate effect on the metabolic functionality of rumen methanogens whereas its impact on microbiome assemblage, specifically methanogen abundance, is delayed.
Conclusions: The methane reducing effect of A. taxiformis during rumen fermentation makes this macroalgae a promising candidate as a biotic methane mitigation strategy for dairy cattle. But its effect in-vivo (i.e. in dairy cattle) remains to be investigated in animal trials. Furthermore, to obtain a holistic understanding of the biochemistry responsible for the significant reduction of methane, gene expression profiles of the rumen microbiome and the host animal are warranted.
Keywords: 16S rRNA community profiling; Asparagopsis taxiformis; Feed supplementation; Greenhouse gas mitigation; In-vitro rumen fermentation; Macroalgae; Rumen microbiome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Smith PM, Bustamante H, Ahammad H, Clark H, Dong EA, Elsiddig H, Haberl R, Harper J, House M, Jafari O, Masera C, Mbow NH, Ravindranath CW, Rice C, Robledo Abad A, Romanovskaya F, Sperling F, Tubiello F. Agriculture, Forestry and Other Land Use (AFOLU) 2013. In: Climate Change: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, and New York: Cambridge University Press; 2013. https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_chapter11.pdf. Accessed 15 Mar 2018.
-
- Myhre G, Shindell D, Bréon F-M, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura T, Zhang H. Anthropogenic and Natural Radiative Forcing 2013. In: Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate. Cambridge and New York: Cambridge University Press; 2013. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf. Accessed 15 Mar 2018.
-
- National Academies of Science Engineering and Medicine (NASEM) Improving characterization of Anthropogenic methane emissions in the United States. Washington: The National Academies Press; 2018. - PubMed
-
- Czerkawski JW. An introduction to rumen studies. 1st. ed. Oxford Oxfordshire: Pergamon Press; 1986.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
