Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub)species
- PMID: 33499982
- PMCID: PMC7807809
- DOI: 10.1186/s42523-020-0023-1
Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub)species
Abstract
Background: Equine gut microbiology studies to date have primarily focused on horses and ponies, which represent only one of the eight extant equine species. This is despite asses and mules comprising almost half of the world's domesticated equines, and donkeys being superior to horses/ponies in their ability to degrade dietary fiber. Limited attention has also been given to commensal anaerobic fungi and archaea even though anaerobic fungi are potent fiber degrading organisms, the activity of which is enhanced by methanogenic archaea. Therefore, the objective of this study was to broaden the current knowledge of bacterial, anaerobic fungal and archaeal diversity of the equine fecal microbiota to multiple species of equines. Core taxa shared by all the equine fecal samples (n = 70) were determined and an overview given of the microbiota across different equine types (horse, donkey, horse × donkey and zebra).
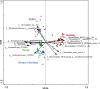
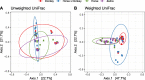
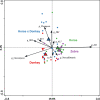
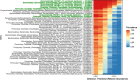
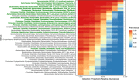
Results: Equine type was associated with differences in both fecal microbial concentrations and community composition. Donkey was generally most distinct from the other equine types, with horse and zebra not differing. Despite this, a common bacterial core of eight OTUs (out of 2070) and 16 genus level groupings (out of 231) was found in all the fecal samples. This bacterial core represented a much larger proportion of the equine fecal microbiota than previously reported, primarily due to the detection of predominant core taxa belonging to the phyla Kiritimatiellaeota (formerly Verrucomicrobia subdivision 5) and Spirochaetes. The majority of the core bacterial taxa lack cultured representation. Archaea and anaerobic fungi were present in all animals, however, no core taxon was detected for either despite several taxa being prevalent and predominant.
Conclusions: Whilst differences were observed between equine types, a core fecal microbiota existed across all the equines. This core was composed primarily of a few predominant bacterial taxa, the majority of which are novel and lack cultured representation. The lack of microbial cultures representing the predominant taxa needs to be addressed, as their availability is essential to gain fundamental knowledge of the microbial functions that underpin the equine hindgut ecosystem.
Keywords: Anaerobic fungi; Archaea; Bacteria; Barcoded amplicon sequencing; Donkey; Feces; Hinny; Horse; Mule; Zebra.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Costa MC, Arroyo LG, Allen-Vercoe E, Stämpfli HR, Kim PT, Sturgeon A, et al. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS One. 2012;7:e41484. doi: 10.1371/journal.pone.0041484. - DOI - PMC - PubMed
-
- Rodriguez C, Taminiau B, Brévers B, Avesani V, Van Broeck J, Leroux A, et al. Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of Clostridium difficile at hospital admission. BMC Microbiol. 2015;15:181. doi: 10.1186/s12866-015-0514-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
