TROAP switches DYRK1 activity to drive hepatocellular carcinoma progression
- PMID: 33500384
- PMCID: PMC7838256
- DOI: 10.1038/s41419-021-03422-3
TROAP switches DYRK1 activity to drive hepatocellular carcinoma progression
Abstract
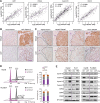
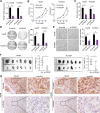
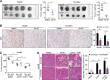
Hepatocellular carcinoma (HCC) is one of the common malignancy and lacks effective therapeutic targets. Here, we demonstrated that ectopic expression of trophinin-associated protein (TROAP) dramatically drove HCC cell growth assessed by foci formation in monolayer culture, colony formation in soft agar and orthotopic liver transplantation in nude mice. Inversely, silencing TROAP expression with short-hairpin RNA attenuated the malignant proliferation of HCC cells in vitro and in vivo. Next, mechanistic investigation revealed that TROAP directly bound to dual specificity tyrosine phosphorylation regulated kinase 1A/B (DYRK1A/B), resulting in the cytoplasmic retention of proteins DYRK1A/B and promoting cell cycle process via activation of Akt/GSK-3β signaling. Combination of cisplatin with an inhibitor of DYRK1 AZ191 effectively inhibited tumor growth in mouse model for HCC cells with high level of TROAP. Clinically, TROAP was significantly upregulated by miR-142-5p in HCC tissues, which predicted the poor survival of patients with HCC. Therefore, TROAP/DYRK1/Akt axis may be a promising therapeutic target and prognostic indicator for patients with HCC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Downregulated Trophinin-Associated Protein Plays a Critical Role in Human Hepatocellular Carcinoma Through Upregulation of Tumor Cell Growth and Migration.Oncol Res. 2018 Jun 11;26(5):691-701. doi: 10.3727/096504017X15101398724809. Epub 2017 Nov 8. Oncol Res. 2018. PMID: 29117881 Free PMC article.
-
High Trophinin-Associated Protein Expression Is an Independent Predictor of Poor Survival in Liver Cancer.Dig Dis Sci. 2019 Jan;64(1):137-143. doi: 10.1007/s10620-018-5315-x. Epub 2018 Oct 4. Dig Dis Sci. 2019. PMID: 30284652
-
STK17B promotes carcinogenesis and metastasis via AKT/GSK-3β/Snail signaling in hepatocellular carcinoma.Cell Death Dis. 2018 Feb 14;9(2):236. doi: 10.1038/s41419-018-0262-1. Cell Death Dis. 2018. PMID: 29445189 Free PMC article.
-
Circular RNA-ABCB10 suppresses hepatocellular carcinoma progression through upregulating NRP1/ABL2 via sponging miR-340-5p/miR-452-5p.Eur Rev Med Pharmacol Sci. 2020 Mar;24(5):2347-2357. doi: 10.26355/eurrev_202003_20501. Eur Rev Med Pharmacol Sci. 2020. PMID: 32196586
-
JCAD Promotes Progression of Nonalcoholic Steatohepatitis to Liver Cancer by Inhibiting LATS2 Kinase Activity.Cancer Res. 2017 Oct 1;77(19):5287-5300. doi: 10.1158/0008-5472.CAN-17-0229. Epub 2017 Aug 3. Cancer Res. 2017. PMID: 28775168
Cited by
-
The zinc transporter ZIP12 regulates monocrotaline-induced proliferation and migration of pulmonary arterial smooth muscle cells via the AKT/ERK signaling pathways.BMC Pulm Med. 2022 Mar 28;22(1):111. doi: 10.1186/s12890-022-01905-3. BMC Pulm Med. 2022. PMID: 35346134 Free PMC article.
-
Microglia Polarization and Antiglioma Effects Fostered by Dual Cell Membrane-Coated Doxorubicin-Loaded Hexagonal Boron Nitride Nanoflakes.ACS Appl Mater Interfaces. 2023 Dec 20;15(50):58260-58273. doi: 10.1021/acsami.3c17097. Epub 2023 Dec 5. ACS Appl Mater Interfaces. 2023. PMID: 38051559 Free PMC article.
-
DYRK1A suppression attenuates HIF‑1α accumulation and enhances the anti‑liver cancer effects of regorafenib and sorafenib under hypoxic conditions.Int J Oncol. 2022 Apr;60(4):45. doi: 10.3892/ijo.2022.5335. Epub 2022 Mar 4. Int J Oncol. 2022. PMID: 35244188 Free PMC article.
-
Kinome-Wide siRNA Screening Identifies DYRK1B as a Potential Therapeutic Target for Triple-Negative Breast Cancer Cells.Cancers (Basel). 2021 Nov 18;13(22):5779. doi: 10.3390/cancers13225779. Cancers (Basel). 2021. PMID: 34830933 Free PMC article.
-
Insights from the protein interaction Universe of the multifunctional "Goldilocks" kinase DYRK1A.Front Cell Dev Biol. 2023 Oct 12;11:1277537. doi: 10.3389/fcell.2023.1277537. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900285 Free PMC article. Review.
References
-
- Suzuki N, et al. A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells. Proc Natl Acad. Sci. USA. 1998;95:5027–5032. doi: 10.1073/pnas.95.9.5027. - DOI - PMC - PubMed
-
- Ye J, et al. TROAP regulates prostate cancer progression via the WNT3/survivin signalling pathways. Oncol. Rep. 2019;41:1169–1179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

