Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization
- PMID: 33500411
- PMCID: PMC7838415
- DOI: 10.1038/s41467-020-20495-4
Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization
Abstract
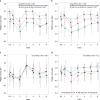
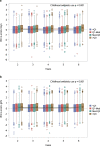
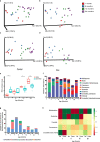
Exposure to antibiotics in the first days of life is thought to affect various physiological aspects of neonatal development. Here, we investigate the long-term impact of antibiotic treatment in the neonatal period and early childhood on child growth in an unselected birth cohort of 12,422 children born at full term. We find significant attenuation of weight and height gain during the first 6 years of life after neonatal antibiotic exposure in boys, but not in girls, after adjusting for potential confounders. In contrast, antibiotic use after the neonatal period but during the first 6 years of life is associated with significantly higher body mass index throughout the study period in both boys and girls. Neonatal antibiotic exposure is associated with significant differences in the gut microbiome, particularly in decreased abundance and diversity of fecal Bifidobacteria until 2 years of age. Finally, we demonstrate that fecal microbiota transplant from antibiotic-exposed children to germ-free male, but not female, mice results in significant growth impairment. Thus, we conclude that neonatal antibiotic exposure is associated with a long-term gut microbiome perturbation and may result in reduced growth in boys during the first six years of life while antibiotic use later in childhood is associated with increased body mass index.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Schrag, S. J. et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics138, 10.1542/peds.2016-2013 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

