Farnesyl dimethyl chromanol targets colon cancer stem cells and prevents colorectal cancer metastasis
- PMID: 33500430
- PMCID: PMC7838198
- DOI: 10.1038/s41598-020-80911-z
Farnesyl dimethyl chromanol targets colon cancer stem cells and prevents colorectal cancer metastasis
Retraction in
-
Retraction Note: Farnesyl dimethyl chromanol targets colon cancer stem cells and prevents colorectal cancer metastasis.Sci Rep. 2025 Nov 10;15(1):39181. doi: 10.1038/s41598-025-27189-1. Sci Rep. 2025. PMID: 41214099 Free PMC article. No abstract available.
Abstract
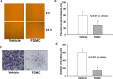
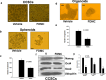
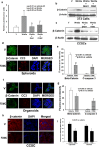
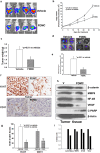
The activation and growth of tumour-initiating cells with stem-like properties in distant organs characterize colorectal cancer (CRC) growth and metastasis. Thus, inhibition of colon cancer stem cell (CCSC) growth holds promise for CRC growth and metastasis prevention. We and others have shown that farnesyl dimethyl chromanol (FDMC) inhibits cancer cell growth and induces apoptosis in vitro and in vivo. We provide the first demonstration that FDMC inhibits CCSC viability, survival, self-renewal (spheroid formation), pluripotent transcription factors (Nanog, Oct4, and Sox2) expression, organoids formation, and Wnt/β-catenin signalling, as evidenced by comparisons with vehicle-treated controls. In addition, FDMC inhibits CCSC migration, invasion, inflammation (NF-kB), angiogenesis (vascular endothelial growth factor, VEGF), and metastasis (MMP9), which are critical tumour metastasis processes. Moreover, FDMC induced apoptosis (TUNEL, Annexin V, cleaved caspase 3, and cleaved PARP) in CCSCs and CCSC-derived spheroids and organoids. Finally, in an orthotopic (cecum-injected CCSCs) xenograft metastasis model, we show that FDMC significantly retards CCSC-derived tumour growth (Ki-67); inhibits inflammation (NF-kB), angiogenesis (VEGF and CD31), and β-catenin signalling; and induces apoptosis (cleaved PARP) in tumour tissues and inhibits liver metastasis. In summary, our results demonstrate that FDMC inhibits the CCSC metastatic phenotype and thereby supports investigating its ability to prevent CRC metastases.
Conflict of interest statement
Dr. Malafa is named as an inventor on US Patent “Delta-Tocotrienol Treatment and Prevention of Pancreatic Cancer” (June 26, 2007; OTML docket number 06A069) but does not have financial interest in the companies that have licensed this patent. Further patents are in development. The other authors declare no competing interests.
Figures


References
-
- Siegel, R. L. et al. Colorectal cancer statistics, 2017. CA Cancer J. Clin.67, 177–193. 10.3322/caac.21395 (2017). - PubMed
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin.68, 7–30. 10.3322/caac.21442 (2018). - PubMed
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin.69, 7–34. 10.3322/caac.21551 (2019). - PubMed
-
- DeSantis, C. E. et al. Cancer treatment and survivorship statistics, 2014. CA A Cancer J. Clin.64, 252–271. 10.3322/caac.21235 (2014). - PubMed
-
- Torre, L. A., Siegel, R. L., Ward, E. M. & Jemal, A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol. Biomark. Prev.25, 16–27. 10.1158/1055-9965.EPI-15-0578 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

