Pre- and postmenopausal women have different core urinary microbiota
- PMID: 33500504
- PMCID: PMC7838182
- DOI: 10.1038/s41598-021-81790-8
Pre- and postmenopausal women have different core urinary microbiota
Abstract
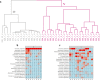
Recent studies suggest that alterations in the female urinary microbiota is associated to development of bladder disease. However, the normal microbiota composition and variation in healthy women are poorly described. Moreover, the effects of hormonal changes on microbiota during menopause is not well understood. The aim of our study was to investigate the urinary microbiota in healthy pre- and postmenopausal women without urinary tract symptoms. Microbiota composition in catheterized urine samples was mapped using 16S rRNA gene sequencing. In total, 41 premenopausal and 42 postmenopausal women were initially included. Samples with first PCR amplification concentration below level of the negative control were excluded, resulting in 34 premenopausal and 20 postmenopausal women included in data analysis. Urine from postmenopausal women showed significantly higher alpha diversity compared to premenopausal women. Lactobacillus was the most abundant bacteria in both groups, however the relative abundance of Lactobacillus accounted for 77.8% in premenopausal versus 42.0% in postmenopausal women. In conclusion, urine from premenopausal mostly presented with Lactobacillus dominated urotypes, whereas urine from postmenopausal women presented a more diverse urinary microbiota with higher abundance of the genera Gardnerella and Prevotella. The clinical and pathophysiological implications of this difference remain to be elucidated.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

