Review on Up-to-Date Status of Candidate Vaccines for COVID-19 Disease
- PMID: 33500636
- PMCID: PMC7826065
- DOI: 10.2147/IDR.S288877
Review on Up-to-Date Status of Candidate Vaccines for COVID-19 Disease
Abstract
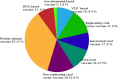
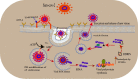
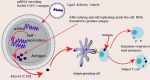
The global pandemic of COVID-19 caused by SARS-CoV-2 continues to spread and poses serious threats to public health and economic stability throughout the world. Thus, to protect the global population, developing safe and effective vaccines is mandatory to control the spread of SARS-CoV-2 pandemic. Since genomic sequences of SARS-CoV-2 and SARS-CoV-1 have similarity and use the same receptor (ACE2), it is important to learn from the development of SARS-CoV-1 vaccines for the development of SARS-CoV-2 vaccines. Normally vaccine development takes 10-15 years but vaccine development against SARS-CoV2 is going on at a very fast pace resulting in almost breakthrough methods of vaccine development by several research institutions. The whole process of vaccine development including clinical trials gets shortened and may be fast tracked to 15-18 months. Global collaborations and increased research efforts among the scientific community have led to more than 214 candidate vaccines globally. The current review highlights the different approaches and technologies used around the world for the design and development of the vaccines and also focuses on the recent status of the SARS-CoV-2 vaccine candidates under development by various institutions to combat the world threat of COVID-19 pandemic.
Keywords: COVID-19; SARS-CoV-2; clinical trials; spike protein; vaccine.
© 2021 Belete.
Conflict of interest statement
The author declares no conflicts of interest for this work.
Figures
References
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int/. Accessed 15 December, 2020.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous