Beyond glucose: alternative sources of energy in glioblastoma
- PMID: 33500708
- PMCID: PMC7797684
- DOI: 10.7150/thno.53506
Beyond glucose: alternative sources of energy in glioblastoma
Abstract
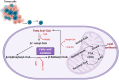
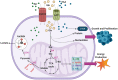
Glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults. With a designation of WHO Grade IV, it is also the most lethal primary brain tumor with a median survival of just 15 months. This is often despite aggressive treatment that includes surgical resection, radiation therapy, and chemotherapy. Based on the poor outcomes and prevalence of the tumor, the demand for innovative therapies continues to represent a pressing issue for clinicians and researchers. In terms of therapies targeting metabolism, the prevalence of the Warburg effect has led to a focus on targeting glucose metabolism to halt tumor progression. While glucose is the dominant source of growth substrate in GBM, a number of unique metabolic pathways are exploited in GBM to meet the increased demand for replication and progression. In this review we aim to explore how metabolites from fatty acid oxidation, the urea cycle, the glutamate-glutamine cycle, and one-carbon metabolism are shunted toward energy producing pathways to meet the high energy demand in GBM. We will also explore how the process of autophagy provides a reservoir of nutrients to support viable tumor cells. By so doing, we aim to establish a foundation of implicated metabolic mechanisms supporting growth and tumorigenesis of GBM within the literature. With the sparse number of therapeutic interventions specifically targeting metabolic pathways in GBM, we hope that this review expands further insight into the development of novel treatment modalities.
Keywords: arginine; autophagy; fatty acids; glioblastoma; glutamine; metabolism.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

