Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel
- PMID: 33500735
- PMCID: PMC7797698
- DOI: 10.7150/thno.46460
Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel
Abstract
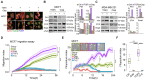
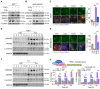
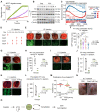
Cancer growth is usually accompanied by metastasis which kills most cancer patients. Here we aim to study the effect of cisplatin at different doses on breast cancer growth and metastasis. Methods: We used cisplatin to treat breast cancer cells, then detected the migration of cells and the changes of epithelial-mesenchymal transition (EMT) markers by migration assay, Western blot, and immunofluorescent staining. Next, we analyzed the changes of RNA expression of genes by RNA-seq and confirmed the binding of activating transcription factor 3 (ATF3) to cytoskeleton related genes by ChIP-seq. Thereafter, we combined cisplatin and paclitaxel in a neoadjuvant setting to treat xenograft mouse models. Furthermore, we analyzed the association of disease prognosis with cytoskeletal genes and ATF3 by clinical data analysis. Results: When administered at a higher dose (6 mg/kg), cisplatin inhibits both cancer growth and metastasis, yet with strong side effects, whereas a lower dose (2 mg/kg) cisplatin blocks cancer metastasis without obvious killing effects. Cisplatin inhibits cancer metastasis through blocking early steps of EMT. It antagonizes transforming growth factor beta (TGFβ) signaling through suppressing transcription of many genes involved in cytoskeleton reorganization and filopodia formation which occur early in EMT and are responsible for cancer metastasis. Mechanistically, TGFβ and fibronectin-1 (FN1) constitute a positive reciprocal regulation loop that is critical for activating TGFβ/SMAD3 signaling, which is repressed by cisplatin induced expression of ATF3. Furthermore, neoadjuvant administration of cisplatin at 2 mg/kg in conjunction with paclitaxel inhibits cancer growth and blocks metastasis without causing obvious side effects by inhibiting colonization of cancer cells in the target organs. Conclusion: Thus, cisplatin prevents breast cancer metastasis through blocking early EMT, and the combination of cisplatin and paclitaxel represents a promising therapy for killing breast cancer and blocking tumor metastasis.
Keywords: TGFβ; cisplatin; metastasis; neoadjuvant therapy; paclitaxel.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

