Retrospective analysis of hemispheric structural network change as a function of location and size of glioma
- PMID: 33501423
- PMCID: PMC7811759
- DOI: 10.1093/braincomms/fcaa216
Retrospective analysis of hemispheric structural network change as a function of location and size of glioma
Abstract
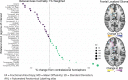
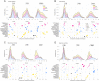
Gliomas are neoplasms that arise from glial cell origin and represent the largest fraction of primary malignant brain tumours (77%). These highly infiltrative malignant cell clusters modify brain structure and function through expansion, invasion and intratumoral modification. Depending on the growth rate of the tumour, location and degree of expansion, functional reorganization may not lead to overt changes in behaviour despite significant cerebral adaptation. Studies in simulated lesion models and in patients with stroke reveal both local and distal functional disturbances, using measures of anatomical brain networks. Investigations over the last two decades have sought to use diffusion tensor imaging tractography data in the context of intracranial tumours to improve surgical planning, intraoperative functional localization, and post-operative interpretation of functional change. In this study, we used diffusion tensor imaging tractography to assess the impact of tumour location on the white matter structural network. To better understand how various lobe localized gliomas impact the topology underlying efficiency of information transfer between brain regions, we identified the major alterations in brain network connectivity patterns between the ipsilesional versus contralesional hemispheres in patients with gliomas localized to the frontal, parietal or temporal lobe. Results were indicative of altered network efficiency and the role of specific brain regions unique to different lobe localized gliomas. This work draws attention to connections and brain regions which have shared structural susceptibility in frontal, parietal and temporal lobe glioma cases. This study also provides a preliminary anatomical basis for understanding which affected white matter pathways may contribute to preoperative patient symptomology.
Keywords: DTI; glioma; graph network; structural connectivity.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Anderson TW. Anderson–darling tests of goodness-of-fit In: Lovric M, editor. International encyclopedia of statistical science. Berlin Heidelberg: Springer, 2011. p. 52–54.
-
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34: 51–61. - PubMed
LinkOut - more resources
Full Text Sources
