This is a preprint.
Cortical Mechanisms of Visual Hypersensitivity in Women at Risk for Chronic Pelvic Pain
- PMID: 33501463
- PMCID: PMC7836135
- DOI: 10.1101/2020.12.03.20242032
Cortical Mechanisms of Visual Hypersensitivity in Women at Risk for Chronic Pelvic Pain
Update in
-
Cortical mechanisms of visual hypersensitivity in women at risk for chronic pelvic pain.Pain. 2022 Jun 1;163(6):1035-1048. doi: 10.1097/j.pain.0000000000002469. Epub 2021 Aug 27. Pain. 2022. PMID: 34510138 Free PMC article.
Abstract
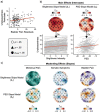
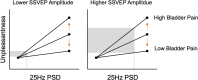
Multisensory hypersensitivity (MSH), which refers to persistent discomfort across sensory modalities, is a risk factor for chronic pain. Developing a better understanding of the neural contributions of disparate sensory systems to MSH may clarify its role in the development of chronic pain. We recruited a cohort of women ( n =147) enriched with participants with menstrual pain at risk for developing chronic pain. Visual sensitivity was measured using a periodic pattern-reversal stimulus during EEG. Self-reported visual unpleasantness ratings were also recorded. Bladder pain sensitivity was evaluated with an experimental bladder-filling task associated with early clinical symptoms of chronic pelvic pain. Visual stimulation induced unpleasantness was associated with bladder pain and evoked primary visual cortex excitation; however, the relationship between unpleasantness and cortical excitation was moderated by bladder pain. Thus, future studies aimed at reversing the progression of MSH into chronic pain should prioritize targeting of cortical mechanisms responsible for maladaptive sensory input integration.
Figures



References
-
- Abrams P., Cardozo L., Fall M., Griffiths D., Rosier P., Ulmsten U., van Kerrebroeck P., Victor A., & Wein A. (2002). The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. American Journal of Obstetrics and Gynecology, 187(1), 116–126. - PubMed
-
- Arendt-Nielsen L., Morlion B., Perrot S., Dahan A., Dickenson A., Kress H. G., Wells C., Bouhassira D., & Drewes A. M. (2017). Assessment and manifestation of central sensitisation across different chronic pain conditions. 26. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
